Exercise-mediated reinnervation of skeletal muscle in elderly people: An update
HTML: 190
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
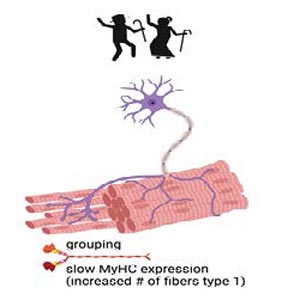
Sarcopenia is defined by the loss of muscle mass and function. In aging sarcopenia is due to mild chronic inflammation but also to fiber-intrinsic defects, such as mitochondrial dysfunction. Age-related sarcopenia is associated with physical disability and lowered quality of life. In addition to skeletal muscle, the nervous tissue is also affected in elderly people. With aging, type 2 fast fibers preferentially undergo denervation and are reinnervated by slow-twitch motor neurons. They spread forming new neuro-muscular junctions with the denervated fibers: the result is an increased proportion of slow fibers that group together since they are associated in the same motor unit. Grouping and fiber type shifting are indeed major histological features of aging skeletal muscle. Exercise has been proposed as an intervention for age-related sarcopenia due to its numerous beneficial effects on muscle mechanical and biochemical features. In 2013, a precursor study in humans was published in the European Journal of Translation Myology (formerly known as Basic and Applied Myology), highlighting the occurrence of reinnervation in the musculature of aged, exercise-trained individuals as compared to the matching control. This paper, entitled «Reinnervation of Vastus lateralis is increased significantly in seniors (70-years old) with a lifelong history of high-level exercise», is now being reprinted for the second issue of the «Ejtm Seminal Paper Series». In this short review we discuss those results in the light of the most recent advances confirming the occurrence of exercise-mediated reinnervation, ultimately preserving muscle structure and function in elderly people who exercise.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.
Similar Articles
- Dario Coletti, Chemotherapy-induced muscle wasting: an update , European Journal of Translational Myology: Vol. 28 No. 2 (2018)
- Ugo Carraro, Exciting perspectives for Translational Myology in the Abstracts of the 2018Spring PaduaMuscleDays: Giovanni Salviati Memorial – Chapter II - Abstracts of March 15, 2018 , European Journal of Translational Myology: Vol. 28 No. 1 (2018)
- Sascha Sajer, Mobility disorders and pain, interrelations that need new research concepts and advanced clinical commitments , European Journal of Translational Myology: Vol 27, No 4 (2017)
- Bianca Maria Scicchitano, Gigliola Sica, Antonio Musarò, Stem cells and tissue niche: two faces of the same coin of muscle regeneration , European Journal of Translational Myology: Vol. 26 No. 4 (2016)
- Barbara Ravara, Valerio Gobbo, Damiana Incendi, Andrea Porzionato, Veronica Macchi, Raffaele De Caro, Dario Coletti, Tiziana Martinello, Marco Patruno, Revisiting the peculiar regional distribution of muscle fiber types in rat Sternomastoid Muscle , European Journal of Translational Myology: Vol. 28 No. 1 (2018)
- Kyle Joseph Edmunds, Magnus K. Gíslason, Iris D. Arnadottir, Andrea Marcante, Francesco Piccione, Paolo Gargiulo, Quantitative Computed Tomography and image analysis for advanced muscle assessment , European Journal of Translational Myology: Vol. 26 No. 2 (2016)
- Sandra Zampieri, Ugo Carraro, Last-minute abstracts of 2023 Padua Days of Muscle and Mobility Medicine (2023 Pdm3) and 2023 Editorial board of EJTM , European Journal of Translational Myology: Vol. 33 No. 1 (2023)
- Barbara Ravara, Walter Giuriati, Maria Chiara Maccarone, Helmut Kern, Stefano Masiero, Ugo Carraro, Optimized progression of Full-Body In-Bed Gym workout: an educational case report , European Journal of Translational Myology: Vol. 33 No. 2 (2023)
- Ugo Carraro, From the Padua Muscle Days, the Basic and Applied Myology and the European Journal of Translational Myology to the A&CM Carraro Foundation for Translational Myology , European Journal of Translational Myology: Vol 27, No 3 (2017)
- The Editors, Abstracts of the Myology Seminar: Are deferrable the mobility impairments in older aging? | Accademia Galileiana di Scienze Lettere ed Arti, Padua, Italy, February 16, 2016 , European Journal of Translational Myology: Vol. 26 No. 1 (2016)
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/ejtm.2022.10416
https://doi.org/10.4081/ejtm.2022.10416



