Muscle function impairment in cancer patients in pre-cachexia stage
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Accepted: 28 March 2020
Authors
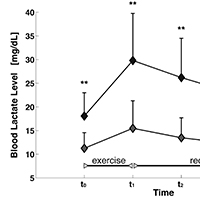
Cancer cachexia has been reported to be directly responsible for at least 20% of cancer deaths. Management of muscle wasting in cancer-associated cachexia appears to be of pivotal importance for survival of patients. In this regard, it would be interesting to identify before its patent appearance eventual functional markers of muscle damage, to plan specific exercise protocols to counteract cachexia. The muscle function of 13 oncologic patients and 15 controls was analyzed through: i) analysis of the oxidative metabolism, indirectly evaluated trough dosage of blood lactate levels before and after a submaximal incremental exercise on a treadmill; ii) analysis of strength and, iii) endurance, in both lower and upper limbs muscles, employing an isokinetic dynamometer. Statistical analyses were carried out to compare the muscle activities between groups. Analysis of oxidative metabolism during the incremental exercise on a treadmill showed that patients performed a shorter exercise than controls. Lactate levels were significantly higher in patients both at baseline and after the task. Muscle strength analysis in patients group showed a reduction of Maximum Voluntary Contraction during the isometric contraction and, a tendency to fatigue during endurance task. Data emerging from this study highlight an impairment of muscle oxidative metabolism in subjects affected by a pre-cachexia stage of cancer. A trend of precocious fatigability and an impairment of muscle strength production were also observed. This evidence underlines the relevance of assessing muscle function in order to develop novel rehabilitative approaches able to counteract motor impairment and eventually to prevent cachexia in these patients.






