Correlation between ETFDH mutations and dysregulation of serum myomiRs in MADD patients
Accepted: 15 February 2020
HTML: 16
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
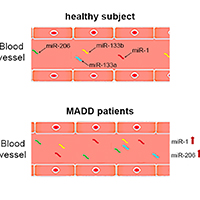
Multiple acyl-CoA dehydrogenase deficiency (MADD) is a rare fatty acids oxidation disorder which is often associated with deficiency of electron transfer flavoprotein dehydrogenase (ETFDH). In this study we reported clinical features and evaluation of expression profile of circulating muscle-specific miRNAs (myomiRs) in two MADD patients carrying different ETFDH gene mutations. Patient 1 was a compound heterozygote for two missense mutations. She showed a late onset MADD clinical phenotype and a significant increase of serum myomiRs. Patient 2, carrying a missense and a frameshift mutation, displayed early onset symptoms and a slight increase of some serum myomiRs.
Merritt JL 2nd, Norris M, Kanungo S. Fatty acid oxidation disorders. Ann Transl Med 2018;6:473. DOI: https://doi.org/10.21037/atm.2018.10.57
Angelini C, Pennisi E, Missaglia S, et al. Metabolic lipid muscle disorders: biomarkers and treatment. Ther Adv Neurol Disord 2019;12:1756286419843359. DOI: https://doi.org/10.1177/1756286419843359
Zhang J, Frerman FE, Kim JJ. Structure of electron transfer flavoprotein-ubiquinone oxidoreductase and electron transfer to the mitochondrial ubiquinone pool. Proc Natl Acad Sci U S A 2006;103:16212–16217. DOI: https://doi.org/10.1073/pnas.0604567103
Grunert SC. Clinical and genetical heterogeneity of late-onset multiple acyl-coenzyme a dehydrogenase deficiency. Orphanet J Rare Dis 2014;9:117. DOI: https://doi.org/10.1186/s13023-014-0117-5
Bhaskaran M, Mohan M. MicroRNAs: History, Biogenesis, and Their Evolving Role in Animal Development and Disease. Vet Pathol 2014;5:759-774. DOI: https://doi.org/10.1177/0300985813502820
Siracusa J, Koulmann N, Banzet S. Circulating myomiRs: a new class of biomarkers to monitor skeletal muscle in physiology and medicine. J Cachexia Sarcopenia Muscle 2018;9:20‐27. DOI: https://doi.org/10.1002/jcsm.12227
Vickers KC, Palmisano BT, Shoucri BM, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoprotein. Nat Cell Biol 2011;13:423-433. DOI: https://doi.org/10.1038/ncb2210
Horak M, Novak J, Bienertova-Vasku J. Muscle-specific microRNAs in skeletal muscle development. Dev Biol 2016;410:1–13. DOI: https://doi.org/10.1016/j.ydbio.2015.12.013
Mitchelson KR, Qin WY. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. World J Biol Chem 2015;6:162–208. DOI: https://doi.org/10.4331/wjbc.v6.i3.162
Cacchiarelli D, Legnini I, Martone J, et al. miRNAs as serum biomarkers for Duchenne muscular dystrophy. EMBO Mol Med 2011;3:258‐265. DOI: https://doi.org/10.1002/emmm.201100133
Pegoraro V, Missaglia S, Marozzo R, et al. MiRNAs as biomarkers of phenotype in neutral lipid storage disease with myopathy. Muscle Nerve 2020;61:253-257. DOI: https://doi.org/10.1002/mus.26761
Angelini C, Tavian D, Missaglia S. Heterogeneous Phenotypes in Lipid Storage Myopathy Due to ETFDH Gene Mutations. JIMD Rep. 2018;38:33-40. DOI: https://doi.org/10.1007/8904_2017_27
Missaglia S, Tavian D, Moro L, et al. Characterization of two ETFDH mutations in a novel case of riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Lipids Health Dis 2018;17:254. DOI: https://doi.org/10.1186/s12944-018-0903-5
Townley-Tilson WHD, Callis TE, Wang D. MicroRNAs 1, 133, and 206: Critical factors of skeletal and cardiac muscle development, function, and disease. Int J Biochem Cell Biol 2010;42:1252–1255 DOI: https://doi.org/10.1016/j.biocel.2009.03.002
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/ejtm.2019.8880
https://doi.org/10.4081/ejtm.2019.8880



