Angiotensin-(1-7) improves skeletal muscle regeneration
Accepted: 21 November 2023
HTML: 3
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
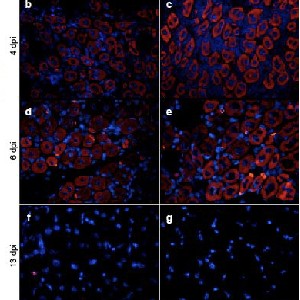
Skeletal muscle possesses regenerative potential via satellite cells, compromised in muscular dystrophies leading to fibrosis and fat infiltration. Angiotensin II (Ang-II) is commonly associated with pathological states. In contrast, Angiotensin (1-7) [Ang-(1-7)] counters Ang-II, acting via the Mas receptor. While Ang-II affects skeletal muscle regeneration, the influence of Ang-(1-7) remains to be elucidated. Therefore, this study aims to investigate the role of Ang-(1-7) in skeletal muscle regeneration. C2C12 cells were differentiated in the absence or presence of 10 nM of Ang-(1-7). The diameter of myotubes and protein levels of myogenin and myosin heavy chain (MHC) were determined. C57BL/6 WT male mice 16-18 weeks old) were randomly assigned to injury-vehicle, injury-Ang-(1-7), and control groups. Ang-(1-7) was administered via osmotic pumps, and muscle injury was induced by injecting barium chloride to assess muscle regeneration through histological analyses. Moreover, embryonic myosin (eMHC) and myogenin protein levels were evaluated. C2C12 myotubes incubated with Ang-(1-7) showed larger diameters than the untreated group and increased myogenin and MHC protein levels during differentiation. Ang-(1-7) administration enhances regeneration by promoting a larger diameter of new muscle fibers. Furthermore, higher numbers of eMHC (+) fibers were observed in the injured-Ang-(1-7), which also had a larger diameter. Moreover, eMHC and myogenin protein levels were elevated, supporting enhanced regeneration due to Ang-(1-7) administration. Ang-(1-7) effectively promotes differentiation in vitroand improves muscle regeneration in the context of injuries, with potential implications for treating muscle-related disorders.
Smith JA, Murach KA, Dyar KA, Zierath JR. Exercise metabolism and adaptation in skeletal muscle. Nature Reviews Molecular Cell Biology. 2023:1-26. DOI: https://doi.org/10.1038/s41580-023-00606-x
Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93(1):23-67. PubMed PMID: 23303905; PubMed Central PMCID: PMCPMC4073943. DOI: https://doi.org/10.1152/physrev.00043.2011
Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA. Satellite Cells and Skeletal Muscle Regeneration. Compr Physiol. 2015;5(3):1027-59. Epub 2015/07/04. PubMed PMID: 26140708. DOI: https://doi.org/10.1002/cphy.c140068
Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143(7):1059-71. PubMed PMID: 21145579; PubMed Central PMCID: PMCPMC3025608. DOI: https://doi.org/10.1016/j.cell.2010.11.039
Schiaffino S, Partridge T. Skeletal muscle repair and regeneration: Springer Science & Business Media; 2008.
Cabello-Verrugio C, Morales MG, Rivera JC, Cabrera D, Simon F. Renin-angiotensin system: an old player with novel functions in skeletal muscle. Med Res Rev. 2015;35(3):437-63. Epub 2015/03/13. PubMed PMID: 25764065. DOI: https://doi.org/10.1002/med.21343
Cabello-Verrugio C, Morales MG, Cabrera D, Vio CP, Brandan E. Angiotensin II receptor type 1 blockade decreases CTGF/CCN2-mediated damage and fibrosis in normal and dystrophic skeletal muscles. J Cell Mol Med. 2012;16(4):752-64. Epub 2011/06/08. PubMed PMID: 21645240; PubMed Central PMCID: PMCPMC3822846. DOI: https://doi.org/10.1111/j.1582-4934.2011.01354.x
Cisternas F, Morales MG, Meneses C, Simon F, Brandan E, Abrigo J, Vazquez Y, Cabello-Verrugio C. Angiotensin-(1-7) decreases skeletal muscle atrophy induced by angiotensin II through a Mas receptor-dependent mechanism. Clin Sci (Lond). 2015 Mar;128(5):307-19. PMID: 25222828..
Meneses C, Morales MG, Abrigo J, Simon F, Brandan E, Cabello-Verrugio C. The angiotensin-(1-7)/Mas axis reduces myonuclear apoptosis during recovery from angiotensin II-induced skeletal muscle atrophy in mice. Pflugers Arch. 2015;467(9):1975-84. PubMed PMID: 25292283. DOI: https://doi.org/10.1007/s00424-014-1617-9
Delafontaine P, Yoshida T. The Renin-Angiotensin System and the Biology of Skeletal Muscle: Mechanisms of Muscle Wasting in Chronic Disease States. Trans Am Clin Climatol Assoc. 2016;127:245-58. Epub 2017/01/10. PubMed PMID: 28066057; PubMed Central PMCID: PMCPMC5216488.
Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J Endocrinol. 2013;216(2):R1-R17. PubMed PMID: 23092879. DOI: https://doi.org/10.1530/JOE-12-0341
Cisternas F, Morales MG, Meneses C, Simon F, Brandan E, Abrigo J, Vazquez Y, Cabello-Verrugio C. Angiotensin-(1-7) decreases skeletal muscle atrophy induced by angiotensin II through a Mas receptor-dependent mechanism. Clin Sci (Lond). 2015 Mar;128(5):307-19. PMID: 25222828.. DOI: https://doi.org/10.1042/CS20140215
Bedair HS, Karthikeyan T, Quintero A, Li Y, Huard J. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. The American journal of sports medicine. 2008;36(8):1548-54. DOI: https://doi.org/10.1177/0363546508315470
Yoshida T, Galvez S, Tiwari S, Rezk BM, Semprun-Prieto L, Higashi Y, Sukhanov S, Yablonka-Reuveni Z, Delafontaine P. Angiotensin II inhibits satellite cell proliferation and prevents skeletal muscle regeneration. J Biol Chem. 2013 Aug 16;288(33):23823-32. Epub 2013 Jul 6. PMID: 23831688; PMCID: PMC3745329.. DOI: https://doi.org/10.1074/jbc.M112.449074
Yoshida T, Huq TS, Delafontaine P. Angiotensin type 2 receptor signaling in satellite cells potentiates skeletal muscle regeneration. J Biol Chem. 2014;289(38):26239-48. PubMed PMID: 25112871; PubMed Central PMCID: PMCPMC4176198. DOI: https://doi.org/10.1074/jbc.M114.585521
Abrigo J, Simon F, Cabrera D, Vilos C, Cabello-Verrugio C. Combined Administration of Andrographolide and Angiotensin- (1-7) Synergically Increases the Muscle Function and Strength in Aged Mice. Curr Mol Med. 2022;22(10):908-18. PubMed PMID: 34875988. DOI: https://doi.org/10.2174/1566524021666211207112106
Casar JC, Cabello-Verrugio C, Olguin H, Aldunate R, Inestrosa NC, Brandan E. Heparan sulfate proteoglycans are increased during skeletal muscle regeneration: requirement of syndecan-3 for successful fiber formation. J Cell Sci. 2004;117(Pt 1):73-84. PubMed PMID: 14627628. DOI: https://doi.org/10.1242/jcs.00828
Abrigo J, Olguín H, Tacchi F, Orozco-Aguilar J, Valero-Breton M, Soto J, Castro-Sepúlveda M, Elorza AA, Simon F, Cabello-Verrugio C. Cholic and deoxycholic acids induce mitochondrial dysfunction, impaired biogenesis and autophagic flux in skeletal muscle cells. Biol Res. 2023 Jun 8;56(1):30. PMID: 37291645; PMCID: PMC10249330. DOI: https://doi.org/10.1186/s40659-023-00436-3
Aravena J, Abrigo J, Gonzalez F, Aguirre F, Gonzalez A, Simon F, Cabello-Verrugio C. Angiotensin (1-7) Decreases Myostatin-Induced NF-κB Signaling and Skeletal Muscle Atrophy. Int J Mol Sci. 2020 Feb 10;21(3):1167. PMID: 32050585; PMCID: PMC7037856..
Angelini C, Pennisi E, Missaglia S, Tavian D. Metabolic lipid muscle disorders: biomarkers and treatment. Ther Adv Neurol Disord. 2019;12:1756286419843359. PubMed PMID: 31040882; PubMed Central PMCID: PMCPMC6477769. DOI: https://doi.org/10.1177/1756286419843359
Carraro U, Kern H, Gava P, Hofer C, Loefler S, Gargiulo P, Mosole S, Zampieri S, Gobbo V, Ravara B, Piccione F, Marcante A, Baba A, Schils S, Pond A, Gava F. Biology of Muscle Atrophy and of its Recovery by FES in Aging and Mobility Impairments: Roots and By-Products. Eur J Transl Myol. 2015 Aug 25;25(4):221-30. PMID: 26913160; PMCID: PMC4748978. DOI: https://doi.org/10.4081/ejtm.2015.5272
Carraro U, Kern H. Severely Atrophic Human Muscle Fibers With Nuclear Misplacement Survive Many Years of Permanent Denervation. Eur J Transl Myol. 2016;26(2):5894. PubMed PMID: 27478559; PubMed Central PMCID: PMCPMC4942702. DOI: https://doi.org/10.4081/ejtm.2016.5894
Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. JBJS. 2002;84(5):822-32. DOI: https://doi.org/10.2106/00004623-200205000-00022
Lin YA, Li YR, Chang YC, Hsu MC, Chen ST. Activation of IGF-1 pathway and suppression of atrophy related genes are involved in Epimedium extract (icariin) promoted C2C12 myotube hypertrophy. Sci Rep. 2021;11(1):10790. PubMed PMID: 34031457; PubMed Central PMCID: PMCPMC8144409. DOI: https://doi.org/10.1038/s41598-021-89039-0
Kitakaze T, Sakamoto T, Kitano T, Inoue N, Sugihara F, Harada N, Yamaji R. The collagen derived dipeptide hydroxyprolyl-glycine promotes C2C12 myoblast differentiation and myotube hypertrophy. Biochem Biophys Res Commun. 2016 Sep 23;478(3):1292-7. Epub 2016 Aug 21. PMID: 27553280. DOI: https://doi.org/10.1016/j.bbrc.2016.08.114
Schmalbruch H. The morphology of regeneration of skeletal muscles in the rat. Tissue Cell. 1976;8(4):673-92. Epub 1976/01/01. PubMed PMID: 1020021. DOI: https://doi.org/10.1016/0040-8166(76)90039-2
Khalil R. Ubiquitin-Proteasome Pathway and Muscle Atrophy. Adv Exp Med Biol. 2018;1088:235-48. Epub 2018/11/06. PubMed PMID: 30390254. DOI: https://doi.org/10.1007/978-981-13-1435-3_10
van der Velden JL, Langen RC, Kelders MC, Willems J, Wouters EF, Janssen-Heininger YM, Schols AM. Myogenic differentiation during regrowth of atrophied skeletal muscle is associated with inactivation of GSK-3beta. Am J Physiol Cell Physiol. 2007 May;292(5):C1636-44. Epub 2006 Dec 13. PMID: 17166938. DOI: https://doi.org/10.1152/ajpcell.00504.2006
Morales MG, Abrigo J, Acuña MJ, Santos RA, Bader M, Brandan E, Simon F, Olguin H, Cabrera D, Cabello-Verrugio C. Angiotensin-(1-7) attenuates disuse skeletal muscle atrophy in mice via its receptor, Mas. Dis Model Mech. 2016 Apr;9(4):441-9. Epub 2016 Feb 5. PMID: 26851244; PMCID: PMC4852504. DOI: https://doi.org/10.1242/dmm.023390
Hernández-Hernández JM, García-González EG, Brun CE, Rudnicki MA, editors. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Seminars in cell & developmental biology; 2017: Elsevier. DOI: https://doi.org/10.1016/j.semcdb.2017.11.010
Zambelli V, Sigurtà A, Rizzi L, Zucca L, Delvecchio P, Bresciani E, Torsello A, Bellani G. Angiotensin-(1-7) exerts a protective action in a rat model of ventilator-induced diaphragmatic dysfunction. Intensive Care Med Exp. 2019 Jan 18;7(1):8. PMID: 30659381; PMCID: PMC6338614. DOI: https://doi.org/10.1186/s40635-018-0218-x
L'honoré A, Commère PH, Negroni E, Pallafacchina G, Friguet B, Drouin J, Buckingham M, Montarras D. The role of Pitx2 and Pitx3 in muscle stem cells gives new insights into P38α MAP kinase and redox regulation of muscle regeneration. Elife. 2018 Aug 14;7:e32991. PMID: 30106373; PMCID: PMC6191287.. DOI: https://doi.org/10.7554/eLife.32991
Pomies P, Blaquiere M, Maury J, Mercier J, Gouzi F, Hayot M. Involvement of the FoxO1/MuRF1/Atrogin-1 Signaling Pathway in the Oxidative Stress-Induced Atrophy of Cultured Chronic Obstructive Pulmonary Disease Myotubes. PLoS One. 2016;11(8):e0160092. PubMed PMID: 27526027; PubMed Central PMCID: PMCPMC4987766. DOI: https://doi.org/10.1371/journal.pone.0160092
Abrigo J, Simon F, Cabrera D, Cabello-Verrugio C. Angiotensin-(1-7) Prevents Skeletal Muscle Atrophy Induced by Transforming Growth Factor Type Beta (TGF-beta) via Mas Receptor Activation. Cell Physiol Biochem. 2016;40(1-2):27-38. Epub 2016/11/15. PubMed PMID: 27842312. DOI: https://doi.org/10.1159/000452522
Forcina L, Cosentino M, Musarò A. Mechanisms regulating muscle regeneration: insights into the interrelated and time-dependent phases of tissue healing. Cells. 2020;9(5):1297. DOI: https://doi.org/10.3390/cells9051297
Morales MG, Olguín H, Di Capua G, Brandan E, Simon F, Cabello-Verrugio C. Endotoxin-induced skeletal muscle wasting is prevented by angiotensin-(1–7) through a p38 MAPK-dependent mechanism. Clinical Science. 2015;129(6):461-76. DOI: https://doi.org/10.1042/CS20140840
Aravena J, Abrigo J, Gonzalez F, Aguirre F, Gonzalez A, Simon F, Cabello-Verrugio C. Angiotensin (1-7) Decreases Myostatin-Induced NF-κB Signaling and Skeletal Muscle Atrophy. Int J Mol Sci. 2020 Feb 10;21(3):1167. PMID: 32050585; PMCID: PMC7037856.. DOI: https://doi.org/10.3390/ijms21031167
Acuna MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Acuña MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M, Muñoz-Canoves P, Santos RA, Cabello-Verrugio C, Brandan E. Restoration of muscle strength in dystrophic muscle by angiotensin-1-7 through inhibition of TGF-β signalling. Hum Mol Genet. 2014 Mar 1;23(5):1237-49. Epub 2013 Oct 24. PMID: 24163134. DOI: https://doi.org/10.1093/hmg/ddt514
Gironacci MM, Adamo HP, Corradi G, Santos RA, Ortiz P, Carretero OA. Angiotensin (1-7) induces MAS receptor internalization. Hypertension. 2011;58(2):176-81. PubMed PMID: 21670420; PubMed Central PMCID: PMCPMC3141282. DOI: https://doi.org/10.1161/HYPERTENSIONAHA.111.173344
Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601-29. doi: 10.1146/annurev.pharmtox.48.113006.094646. PubMed PMID: 17995450; PubMed Central PMCID: PMCPMC2869288. DOI: https://doi.org/10.1146/annurev.pharmtox.48.113006.094646
Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1-7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension. 2005;45(5):960-6. PubMed PMID: 15767466. DOI: https://doi.org/10.1161/01.HYP.0000160325.59323.b8
Copyright (c) 2023 the Author(s)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/ejtm.2023.12037
https://doi.org/10.4081/ejtm.2023.12037



