Immediate to short-term inflammatory response to biomaterial implanted in calvarium of mice
Accepted: 21 August 2022
HTML: 3
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
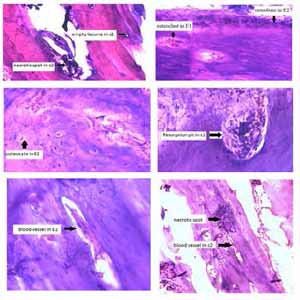
Scaffolds made of biodegradable materials play a very important role in repairing bone defects. Our study was conducted with the aim of investigating inflammation, vascular changes, and tissue necrosis after the placement of 3D printed scaffolds composed of beta-tricalcium phosphate (TCP-β) on the calvarial bone defect of mice. Eight samples of scalp tissue in mice were examined in four groups (one-week control, two-week control, one-week experiment, and two-week experiment). Mice with routine bone defects were selected as the control group and mice with bone defects with β-TCP scaffolds were selected as the experimental group (TCP). The groups were evaluated in terms of inflammatory cells, osteoblast and osteoclast cells, vascular changes, and the number of resorption pit and empty lacuna. The results demonstrated a decrease in inflammatory cells and an increase in osteoclast and osteoblast cells in bone defect sites placed with TCP-β scaffolds (p<0.05). The results of histological staining showed pit resorption and further vascularization in the place of TCP-β scaffolds, but these changes were not statistically significant (p>0.05). Examining the number of empty lacunae in the bone defect site showed that TCP-β could significantly reduce the number of these lacunae in the bone defect sites placed with TCP-β scaffolds (p<0.05). 3D printed scaffolds composed of TCP-β that were implanted in bone defect sites were effective in reducing the inflammatory responses, emptying lacunae and increasing bone regeneration.
Zhang Q, Wu W, Qian C, Xiao W, Zhu H, Guo J, et al. Advanced biomaterials for repairing and reconstruction of mandibular defects. Mater Sci Eng C Mater Biol Appl. 2019;103:109858. DOI: https://doi.org/10.1016/j.msec.2019.109858
Malá E, Vejražková E, Bielmeierová J, Jindra M, Vošmik M, Novosad J, et al. [Long Term Monitoring of Nutritional, Clinical Status and Quality of Life in Head and Neck Cancer Patients]. Klin Onkol. 2015;28(3):200-14. DOI: https://doi.org/10.14735/amko2015200
Lopez CD, Diaz-Siso JR, Witek L, Bekisz JM, Cronstein BN, Torroni A, et al. Three dimensionally printed bioactive ceramic scaffold osseoconduction across critical-sized mandibular defects. J Surg Res. 2018;223:115-22. DOI: https://doi.org/10.1016/j.jss.2017.10.027
Bohner M, Santoni BLG, Döbelin N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020;113:23-41. DOI: https://doi.org/10.1016/j.actbio.2020.06.022
Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36 Suppl 3:S20-7. DOI: https://doi.org/10.1016/j.injury.2005.07.029
Almeida CR, Serra T, Oliveira MI, Planell JA, Barbosa MA, Navarro M. Impact of 3-D printed PLA- and chitosan-based scaffolds on human monocyte/macrophage responses: unraveling the effect of 3-D structures on inflammation. Acta Biomater. 2014;10(2):613-22. DOI: https://doi.org/10.1016/j.actbio.2013.10.035
Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86-100. DOI: https://doi.org/10.1016/j.smim.2007.11.004
Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I, et al. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater Sci Eng C Mater Biol Appl. 2017;78:1246-62. DOI: https://doi.org/10.1016/j.msec.2017.05.017
Rubio-Palau J, Prieto-Gundin A, Cazalla AA, Serrano MB, Fructuoso GG, Ferrandis FP, Baró AR. Three-dimensional planning in craniomaxillo facial surgery. Ann Maxillofac Surg. 2016 Jul-Dec;6(2):281-286. DOI: https://doi.org/10.4103/2231-0746.200322
Ishida S, Shibuya Y, Kobayashi M, Komori T. Assessing stomatognathic performance after mandibulectomy according to the method of mandibular reconstruction. Int J Oral Maxillofac Surg. 2015;44(8):948-55. DOI: https://doi.org/10.1016/j.ijom.2015.03.011
Kumar BP, Venkatesh V, Kumar KA, Yadav BY, Mohan SR. Mandibular Reconstruction: Overview. J Maxillofac Oral Surg. 2016;15(4):425-41. DOI: https://doi.org/10.1007/s12663-015-0766-5
Sarkar SK, Lee BT. Hard tissue regeneration using bone substitutes: an update on innovations in materials. Korean J Intern Med. 2015;30(3):279-93. DOI: https://doi.org/10.3904/kjim.2015.30.3.279
Wan C, He Q, Li G. Allogenic peripheral blood derived mesenchymal stem cells (MSCs) enhance bone regeneration in rabbit ulna critical-sized bone defect model. J Orthop Res. 2006;24(4):610-8. DOI: https://doi.org/10.1002/jor.20119
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2-17. DOI: https://doi.org/10.1002/jbmr.1805
Sridharan R, Ryan EJ, Kearney CJ, Kelly DJ, O'Brien FJ. Macrophage Polarization in Response to Collagen Scaffold Stiffness Is Dependent on Cross-Linking Agent Used To Modulate the Stiffness. ACS Biomater Sci Eng. 2019;5(2):544-52. DOI: https://doi.org/10.1021/acsbiomaterials.8b00910
Shi GS, Li YY, Luo YP, Jin JF, Sun YX, Zheng LZ, et al. Bioactive PLGA/tricalcium phosphate scaffolds incorporating phytomolecule icaritin developed for calvarial defect repair in rat model. J Orthop Translat. 2020;24:112-20. DOI: https://doi.org/10.1016/j.jot.2020.05.008
Wang Z, Guo Z, Bai H, Li J, Li X, Chen G, et al. Clinical evaluation of β-TCP in the treatment of lacunar bone defects: a prospective, randomized controlled study. Mater Sci Eng C Mater Biol Appl. 2013;33(4):1894-9. DOI: https://doi.org/10.1016/j.msec.2012.12.041
Mohd N, Razali M, Ghazali MJ, Abu Kasim NH. 3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review. Materials (Basel). 2022;15(7). DOI: https://doi.org/10.3390/ma15072621
19.Bi G, Mo L, Liu S, Zhong X, Yang J, Yuan Z, Chen S, Ren L. DLP printed β-tricalcium phosphate functionalized ceramic scaffolds promoted angiogenesis and osteogenesis in long bone defects. Ceramics International. 2022.24:112-20. DOI: https://doi.org/10.1016/j.ceramint.2022.05.310
Dumas JE, Prieto EM, Zienkiewicz KJ, Guda T, Wenke JC, Bible J, et al. Balancing the rates of new bone formation and polymer degradation enhances healing of weight-bearing allograft/polyurethane composites in rabbit femoral defects. Tissue Eng Part A. 2014;20(1-2):115-29. DOI: https://doi.org/10.1089/ten.tea.2012.0762
Brown BN, Badylak SF. Expanded applications, shifting paradigms and an improved understanding of host-biomaterial interactions. Acta Biomater. 2013;9(2):4948-55. DOI: https://doi.org/10.1016/j.actbio.2012.10.025
Córdova LA, Loi F, Lin TH, Gibon E, Pajarinen J, Nabeshima A, et al. CCL2, CCL5, and IGF-1 participate in the immunomodulation of osteogenesis during M1/M2 transition in vitro. J Biomed Mater Res A. 2017;105(11):3069-76. DOI: https://doi.org/10.1002/jbm.a.36166
Lu LY, Loi F, Nathan K, Lin TH, Pajarinen J, Gibon E, et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J Orthop Res. 2017;35(11):2378-85. DOI: https://doi.org/10.1002/jor.23553
van Well GTJ, Daalderop LA, Wolfs T, Kramer BW. Human perinatal immunity in physiological conditions and during infection. Mol Cell Pediatr. 2017;4(1):4. DOI: https://doi.org/10.1186/s40348-017-0070-1
Al-Maawi S, Orlowska A, Sader R, James Kirkpatrick C, Ghanaati S. In vivo cellular reactions to different biomaterials - Physiological and pathological aspects and their consequences. Semin Immunol. 2017;29:49-61. DOI: https://doi.org/10.1016/j.smim.2017.06.001
Wright HL, McCarthy HS, Middleton J, Marshall MJ. RANK, RANKL and osteoprotegerin in bone biology and disease. Curr Rev Musculoskelet Med. 2009;2(1):56-64. DOI: https://doi.org/10.1007/s12178-009-9046-7
Rotbaum Y, Puiu C, Rittel D, Domingos M. Quasi-static and dynamic in vitro mechanical response of 3D printed scaffolds with tailored pore size and architectures. Mater Sci Eng C Mater Biol Appl. 2019;96:176-82. DOI: https://doi.org/10.1016/j.msec.2018.11.019
Nie W, Gao Y, McCoul DJ, Gillispie GJ, Zhang Y, Liang L, et al. Rapid mineralization of hierarchical poly(l-lactic acid)/ poly(ε-caprolactone) nano-fibrous scaffolds by electrodeposition for bone regeneration. Int J Nanomedicine. 2019;14:3929-41. DOI: https://doi.org/10.2147/IJN.S205194
Diao J, OuYang J, Deng T, Liu X, Feng Y, Zhao N, Mao C, Wang Y. 3D-Plotted Beta-Tricalcium Phosphate Scaffolds with Smaller Pore Sizes Improve In Vivo Bone Regeneration and Biomechanical Properties in a Critical-Sized Calvarial Defect Rat Model. Adv Healthc Mater. 2018 Sep;7(17):e1800441. Epub 2018 Jul 25. DOI: https://doi.org/10.1002/adhm.201800441
Bian W, Li D, Lian Q, Li X, Zhang W, Wang K, Jin Z. Fabrication of a bio‐inspired beta‐Tricalcium phosphate/collagen scaffold based on ceramic stereolithography and gel casting for osteochondral tissue engineering. Rapid Prototyping Journal. 2012:18:49-62. DOI: https://doi.org/10.1108/13552541211193511
Noori A, Ashrafi SJ, Vaez-Ghaemi R, Hatamian-Zaremi A, Webster TJ. A review of fibrin and fibrin composites for bone tissue engineering. Int J Nanomedicine. 2017;12:4937-61. DOI: https://doi.org/10.2147/IJN.S124671
Wang X, Wang Y, Gou W, Lu Q, Peng J, Lu S. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop. 2013;37(12):2491-8. DOI: https://doi.org/10.1007/s00264-013-2059-2
Mercado-Pagán Á E, Stahl AM, Shanjani Y, Yang Y. Vascularization in bone tissue engineering constructs. Ann Biomed Eng. 2015;43(3):718-29. DOI: https://doi.org/10.1007/s10439-015-1253-3
Jiao X, Sun X, Li W, Chu W, Zhang Y, Li Y, Wang Z, Zhou X, Ma J, Xu C, Dai K, Wang J, Gan Y. 3D-Printed β-Tricalcium Phosphate Scaffolds Promote Osteogenic Differentiation of Bone Marrow-Deprived Mesenchymal Stem Cells in an N6-methyladenosine-Dependent Manner. Int J Bioprint. 2022 Mar 22;8(2):544. DOI: https://doi.org/10.18063/ijb.v8i2.544
Copyright (c) 2022 The Authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/ejtm.2022.10785
https://doi.org/10.4081/ejtm.2022.10785



