Motion sickness susceptibility and visually induced motion sickness as diagnostic signs in Parkinson’s disease
Accepted: 6 October 2022
HTML: 9
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
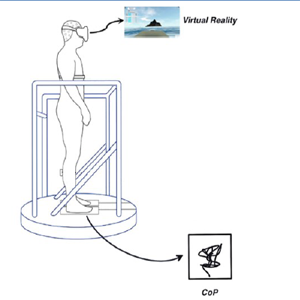
Postural instability and loss of vestibular and somatosensory acuity can be part of the signs encountered in Parkinson’s Disease (PD). Visual dependency is described in PD. These modifications of sensory input hierarchy are predictors of motion sickness (MS). The aim of this study was to assess MS susceptibility and effects of real induced MS in posture. 63 PD patients, whose medication levels (levodopa) reflected the pathology were evaluated, and 27 healthy controls, filled a MS questionnaire; 9 PD patients and 43 healthy controls were assessed by posturography using virtual reality. Drug amount predicted visual MS (p=0.01), but not real induced MS susceptibility. PD patients did not experience postural instability in virtual reality, contrary to healthy controls. Since PD patients do not seem to feel vestibular stimulated MS, they may not rely on vestibular and somatosensory inputs during the stimulation. However, they feel visually induced MS more with increased levodopa drug effect. Levodopa amount can increase visual dependency. The strongest MS predictors must be studied in PD to better understand the effect of visual stimulation and its absence in vestibular stimulation.
Nashner LM, McCollum G. The organization of human postural movements: A formal basis and experimental synthesis. Behavioral and Brain Sciences. Cambridge University Press; 1985; 8(1):135–150. DOI: https://doi.org/10.1017/S0140525X00020008
Mian TS. An Unsupervised Neural Network Feature Selection and 1D Convolution Neural Network Classification for Screening of Parkinsonism. Diagnostics (Basel). 2022 Jul 25;12(8):1796. DOI: https://doi.org/10.3390/diagnostics12081796
Colnat-Coulbois S, Gauchard GC, Maillard L, Barroche G, Vespignani H, Auque J, Perrin PP. Bilateral subthalamic nucleus stimulation improves balance control in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2005 Jun;76(6):780-7. DOI: https://doi.org/10.1136/jnnp.2004.047829
Nallegowda M, Singh U, Handa G, Khanna M, Wadhwa S, Yadav SL, Kumar G, Behari M. Role of sensory input and muscle strength in maintenance of balance, gait, and posture in Parkinson's disease: a pilot study. Am J Phys Med Rehabil. 2004 Dec;83(12):898-908. DOI: https://doi.org/10.1097/01.PHM.0000146505.18244.43
Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J Neurophysiol. 1996 Jun;75(6): 2380-96. DOI: https://doi.org/10.1152/jn.1996.75.6.2380
Zia S, Cody F, O’Boyle D. Joint position sense is impaired by Parkinson’s disease. Ann Neurol. 2000;47(2):218–28. DOI: https://doi.org/10.1002/1531-8249(200002)47:2<218::AID-ANA12>3.0.CO;2-#
Maschke M, Gomez CM, Tuite PJ, Konczak J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain. 2003 Oct;126(Pt 10):2312-22. Epub 2003 Jun 23. DOI: https://doi.org/10.1093/brain/awg230
Teasdale H, Preston E, Waddington G. Proprioception of the Ankle is Impaired in People with Parkinson's Disease. Mov Disord Clin Pract. 2017 Mar 29;4(4):524-528. DOI: https://doi.org/10.1002/mdc3.12464
Konczak J, Corcos DM, Horak F, Poizner H, Shapiro M, Tuite P, Volkmann J, Maschke M. Proprioception and motor control in Parkinson's disease. J Mot Behav. 2009 Nov;41(6):543-52. DOI: https://doi.org/10.3200/35-09-002
Vaugoyeau M, Viel S, Assaiante C, Amblard B, Azulay JP. Impaired vertical postural control and proprioceptive integration deficits in Parkinson's disease. Neuroscience. 2007 May 11;146(2):852-63. Epub 2007 Mar 23. DOI: https://doi.org/10.1016/j.neuroscience.2007.01.052
Smith PF. Vestibular Functions and Parkinson's Disease. Front Neurol. 2018 Dec 11;9:1085. DOI: https://doi.org/10.3389/fneur.2018.01085
Pastor MA, Day BL, Marsden CD. Vestibular induced postural responses in Parkinson's disease. Brain. 1993 Oct;116 ( Pt 5):1177-90. DOI: https://doi.org/10.1093/brain/116.5.1177
de Natale ER, Ginatempo F, Paulus KS, Pes GM, Manca A, Tolu E, Agnetti V, Deriu F. Abnormalities of vestibular-evoked myogenic potentials in idiopathic Parkinson's disease are associated with clinical evidence of brainstem involvement. Neurol Sci. 2015 Jun;36(6):995-1001. Epub 2015 Jan 8. DOI: https://doi.org/10.1007/s10072-014-2054-4
Bertolini G, Wicki A, Baumann CR, Straumann D, Palla A. Impaired tilt perception in Parkinson's disease: a central vestibular integration failure. PLoS One. 2015 Apr 15;10(4):e0124253. DOI: https://doi.org/10.1371/journal.pone.0124253
Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson's disease. Vision Res. 2005 May;45(10):1285-96. Epub 2004 Dec 16. DOI: https://doi.org/10.1016/j.visres.2004.11.006
Halperin O, Israeli-Korn S, Yakubovich S, Hassin-Baer S, Zaidel A. Self-motion perception in Parkinson's disease. Eur J Neurosci. 2021 Apr;53(7):2376-2387. doi: 10.1111/ejn.14716. Epub 2020 Mar 20. DOI: https://doi.org/10.1111/ejn.14716
Colnat-Coulbois S, Gauchard GC, Maillard L, Barroche G, Vespignani H, Auque J, Perrin PP. Management of postural sensory conflict and dynamic balance control in late-stage Parkinson's disease. Neuroscience. 2011 Oct 13;193:363-9. doi: 10.1016/j.neuroscience.2011.04.043. Epub 2011 May 27. DOI: https://doi.org/10.1016/j.neuroscience.2011.04.043
Hwang S, Agada P, Grill S, Kiemel T, Jeka JJ. A central processing sensory deficit with Parkinson's disease. Exp Brain Res. 2016 Aug;234(8):2369-79. doi: 10.1007/s00221-016-4642-4. Epub 2016 Apr 8. DOI: https://doi.org/10.1007/s00221-016-4642-4
Halperin O, Karni R, Israeli-Korn S, Hassin-Baer S, Zaidel A. Overconfidence in visual perception in parkinson's disease. Eur J Neurosci. 2021 Mar;53(6):2027-2039. doi: 10.1111/ejn.15093. Epub 2021 Jan 12. DOI: https://doi.org/10.1111/ejn.15093
Barnett-Cowan M, Dyde RT, Fox SH, Moro E, Hutchison WD, Harris LR. Multisensory determinants of orientation perception in Parkinson's disease. Neuroscience. 2010 Jun 2;167(4):1138-50. doi: 10.1016/j.neuroscience. 2010.02.065. Epub 2010 Mar 4. DOI: https://doi.org/10.1016/j.neuroscience.2010.02.065
Yakubovich S, Israeli-Korn S, Halperin O, Yahalom G, Hassin-Baer S, Zaidel A. Visual self-motion cues are impaired yet overweighted during visual-vestibular integration in Parkinson's disease. Brain Commun. 2020 Mar 31;2(1):fcaa035. doi: 10.1093/braincomms/fcaa035. DOI: https://doi.org/10.1093/braincomms/fcaa035
Azulay JP, Mesure S, Amblard B, Pouget J. Increased visual dependence in Parkinson's disease. Percept Mot Skills. 2002 Dec;95(3 Pt 2):1106-14. doi: 10.2466/pms.2002.95.3f.1106. DOI: https://doi.org/10.2466/pms.2002.95.3f.1106
Hawkins KE, Paul SS, Chiarovano E, Curthoys IS. Using virtual reality to assess vestibulo-visual interaction in people with Parkinson's disease compared to healthy controls. Exp Brain Res. 2021 Dec;239(12):3553-64. doi: 10.1007/s00221-021-06219-0. Epub 2021 Sep 25. DOI: https://doi.org/10.1007/s00221-021-06219-0
Kohl RL. Sensory conflict theory of space motion sickness: an anatomical location for the neuroconflict. Aviat Space Environ Med. 1983 May;54(5):464-5.
Reason JT, Brand JJ. Motion sickness. Oxford, England: Academic Press; 1975. 310 p.
Reason JT. Motion sickness—some theoretical considerations. Int J Man Mach Stud. 1969 Jan;1(1):21–38 doi: 10.1016/S0020-7373(69)80009-X. DOI: https://doi.org/10.1016/S0020-7373(69)80009-X
McCauley ME, Sharkey TJ. Cybersickness: Perception of Self-Motion in Virtual Environments. Presence Teleoperators Virtual Environ. 1992 Aug 1;1(3):311–8. doi: 10.1162/ pres.1992.1.3.311 DOI: https://doi.org/10.1162/pres.1992.1.3.311
Oman CM. A heuristic mathematical model for the dynamics of sensory conflict and motion sickness. Acta Otolaryngol Suppl. 1982;392:1-44. DOI: https://doi.org/10.3109/00016488209108197
Riccio GE, Stoffregen TA. An ecological Theory of Motion Sickness and Postural Instability. Ecol Psychol. 1991 Sep;3(3):195–240. DOI: https://doi.org/10.1207/s15326969eco0303_2
Mittelstaedt JM. Individual predictors of the susceptibility for motion-related sickness: A systematic review. J Vestib Res. 2020;30(3):165-193. doi: 10.3233/VES-200702. DOI: https://doi.org/10.3233/VES-200702
Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, Bottacchi E, Cannas A, Ceravolo G, Ceravolo R, Cicarelli G, Gaglio RM, Giglia RM, Iemolo F, Manfredi M, Meco G, Nicoletti A, Pederzoli M, Petrone A, Pisani A, Pontieri FE, Quatrale R, Ramat S, Scala R, Volpe G, Zappulla S, Bentivoglio AR, Stocchi F, Trianni G, Dotto PD; PRIAMO study group. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord. 2009 Aug 15;24(11):1641-9. doi: 10.1002/mds.22643. DOI: https://doi.org/10.1002/mds.22643
Chaudhuri KR, Odin P, Antonini A, Martinez-Martin P. Parkinson's disease: the non-motor issues. Parkinsonism Relat Disord. 2011 Dec;17(10):717-23. doi: 10.1016/j.parkreldis. 2011.02.018. Epub 2011 Jul 8. DOI: https://doi.org/10.1016/j.parkreldis.2011.02.018
Alexander NB. Postural control in older adults. J Am Geriatr Soc. 1994 Jan;42(1):93-108. doi: 10.1111/j.1532-5415.1994.tb06081.x. DOI: https://doi.org/10.1111/j.1532-5415.1994.tb06081.x
Bloem BR, Beckley DJ, van Dijk JG, Zwinderman AH, Remler MP, Roos RA. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson's disease. Mov Disord. 1996 Sep;11(5):509-21. doi: 10.1002/mds.870110506. DOI: https://doi.org/10.1002/mds.870110506
O'Suilleabhain P, Bullard J, Dewey RB. Proprioception in Parkinson's disease is acutely depressed by dopaminergic medications. J Neurol Neurosurg Psychiatry. 2001 Nov;71(5):607-10. doi: 10.1136/jnnp.71.5.607. DOI: https://doi.org/10.1136/jnnp.71.5.607
Bronte-Stewart HM, Minn AY, Rodrigues K, Buckley EL, Nashner LM. Postural instability in idiopathic Parkinson's disease: the role of medication and unilateral pallidotomy. Brain. 2002 Sep;125(Pt 9):2100-14. doi: 10.1093/brain/awf 207. DOI: https://doi.org/10.1093/brain/awf207
Recenti M, Ricciardi C, Aubonnet R, Picone I, Jacob D, Svansson HÁR, Agnarsdóttir S, Karlsson GH, Baeringsdóttir V, Petersen H, Gargiulo P. Toward Predicting Motion Sickness Using Virtual Reality and a Moving Platform Assessing Brain, Muscles, and Heart Signals. Front Bioeng Biotechnol. 2021 Apr 1;9:635661. doi: 10.3389/fbioe.2021.635661. DOI: https://doi.org/10.3389/fbioe.2021.635661
Jacob D, Unnsteinsdóttir Kristensen IS, Aubonnet R, Recenti M, Donisi L, Ricciardi C, Svansson HÁR, Agnarsdóttir S, Colacino A, Jónsdóttir MK, Kristjánsdóttir H, Sigurjónsdóttir HÁ, Cesarelli M, Eggertsdóttir Claessen LÓ, Hassan M, Petersen H, Gargiulo P. Towards defining biomarkers to evaluate concussions using virtual reality and a moving platform (BioVRSea). Sci Rep. 2022 May 30;12(1):8996. doi: 10.1038/s41598-022-12822-0. DOI: https://doi.org/10.1038/s41598-022-12822-0
Golding JF. Predicting individual differences in motion sickness susceptibility by questionnaire. Personal Individ Differ. 2006 Jul;41(2):237–48. doi: 10.1016/j.paid.2006.01.012 DOI: https://doi.org/10.1016/j.paid.2006.01.012
Bosser G, Caillet G, Gauchard G, Marçon F, Perrin P. Relation between motion sickness susceptibility and vasovagal syncope susceptibility. Brain Res Bull. 2006 Jan 15;68(4):217-26. doi: 10.1016/j.brainresbull. 2005.05.031. Epub 2005 Nov 2. DOI: https://doi.org/10.1016/j.brainresbull.2005.05.031
Longridge NS, Mallinson AI. Visual vestibular mismatch in work-related vestibular injury. Otol Neurotol. 2005 Jul;26(4):691-4. doi: 10.1097/01. mao.0000169637.71064.c6. DOI: https://doi.org/10.1097/01.mao.0000169637.71064.c6
Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967 May;17(5):427-42. doi: 10.1212/wnl.17.5.427. DOI: https://doi.org/10.1212/WNL.17.5.427
Jones JA, Swan JE, Singh G, Kolstad E, Ellis SR. The effects of virtual reality, augmented reality, and motion parallax on egocentric depth perception. Proc 5th Symp Appl Percept Graph Vis. 2008;9–14. DOI: https://doi.org/10.1145/1394281.1394283
Golding JF. Motion sickness susceptibility. Auton Neurosci. 2006 Oct;129(1–2):67–76. doi: 10.1016/ j.paid.2006.01.012 DOI: https://doi.org/10.1016/j.autneu.2006.07.019
Caillet G, Bosser G, Gauchard GC, Chau N, Benamghar L, Perrin PP. Effect of sporting activity practice on susceptibility to motion sickness. Brain Res Bull. 2006 Apr 14;69(3):288-93. doi: 10.1016/j.brainresbull.2006.01.001. Epub 2006 Jan 19. DOI: https://doi.org/10.1016/j.brainresbull.2006.01.001
Owsley C. Aging and vision. Vision Res. 2011;51(13):1610–22. doi: 10.1016/j.visres.2010. 10.020 DOI: https://doi.org/10.1016/j.visres.2010.10.020
Zalewski CK. Aging of the Human Vestibular System. Semin Hear. 2015 Aug;36(3):175-96. doi: 10.1055/s-0035-1555120. DOI: https://doi.org/10.1055/s-0035-1555120
Goble DJ, Coxon JP, Wenderoth N, Van Impe A, Swinnen SP. Proprioceptive sensibility in the elderly: degeneration, functional consequences and plastic-adaptive processes. Neurosci Biobehav Rev. 2009 Mar;33(3):271-8. doi: 10.1016/j.neubio rev.2008.08.012. Epub 2008 Aug 26. DOI: https://doi.org/10.1016/j.neubiorev.2008.08.012
Arns LL, Cerney MM. The relationship between age and incidence of cybersickness among immersive environment users. In: IEEE Proceedings VR 2005 Virtual Reality, 2005. 2005. p. 267–8.
Bos JE, Damala D, Lewis C, Ganguly A, Turan O. Susceptibility to seasickness. Ergonomics. 2007 Jun;50(6):890–901. DOI: https://doi.org/10.1080/00140130701245512
Turner M, Griffin MJ. Motion sickness in public road transport: The relative importance of motion, vision and individual differences. Br J Psychol. 1999;90(4):519–30. doi: 10.1348/000712699161 594 DOI: https://doi.org/10.1348/000712699161594
Lv W, Guan Q, Hu X, Chen J, Jiang H, Zhang L, Fan W. Vestibulo-ocular reflex abnormality in Parkinson's disease detected by video head impulse test. Neurosci Lett. 2017 Sep 14;657:211-214. doi: 10.1016/j.neulet.2017.08.021. Epub 2017 Aug 12. DOI: https://doi.org/10.1016/j.neulet.2017.08.021
Clément G, Reschke MF. Relationship between motion sickness susceptibility and vestibulo-ocular reflex gain and phase. J Vestib Res. 2018;28(3-4):295-304. doi: 10.3233/VES-180632. DOI: https://doi.org/10.3233/VES-180632
Singh NK, Pandey P, Mahesh S. Assessment of otolith function using cervical and ocular vestibular evoked myogenic potentials in individuals with motion sickness. Ergonomics. 2014;57(12):1907-18. doi: 10.1080/00140139. 2014.952683. Epub 2014 Sep 15. DOI: https://doi.org/10.1080/00140139.2014.952683
Edmunds KJ, Petersen H, Hassan M, Yassine S, Olivieri A, Barollo F, Friðriksdóttir R, Edmunds P, Gíslason MK, Fratini A, Gargiulo P. Cortical recruitment and functional dynamics in postural control adaptation and habituation during vibratory proprioceptive stimulation. J Neural Eng. 2019 Apr;16(2):026037. doi: 10.1088/1741-2552/ ab0678. Epub 2019 Feb 12. DOI: https://doi.org/10.1088/1741-2552/ab0678
Barollo F, Frioriksdottir R, Edmunds KJ, Karlsson GH, Svansson HA, Hassan M, Fratini A, Petersen H, Gargiulo P. Postural Control Adaptation and Habituation During Vibratory Proprioceptive Stimulation: An HD-EEG Investigation of Cortical Recruitment and Kinematics. IEEE Trans Neural Syst Rehabil Eng. 2020 Jun;28(6):1381-1388. doi: 10.1109/TNSRE.2020.2988585. Epub 2020 Apr 17. DOI: https://doi.org/10.1109/TNSRE.2020.2988585
Barollo F, Hassan M, Petersen H, Rigoni I, Ramon C, Gargiulo P, Fratini A. Cortical Pathways During Postural Control: New Insights From Functional EEG Source Connectivity. IEEE Trans Neural Syst Rehabil Eng. 2022;30:72-84. doi: 101109/TNSRE.2022.3140888. Epub 2022 Jan 28. DOI: https://doi.org/10.1109/TNSRE.2022.3140888
Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, Grigoletto F, Amaducci L, Inzitari D. Parkinson's disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology. 2000 Nov 14;55(9):1358-63. doi: 10.1212/wnl.55.9. 1358. DOI: https://doi.org/10.1212/WNL.55.9.1358
Jurado-Coronel JC, Cabezas R, Ávila Rodríguez MF, Echeverria V, García-Segura LM, Barreto GE. Sex differences in Parkinson's disease: Features on clinical symptoms, treatment outcome, sexual hormones and genetics. Front Neuroendocrinol. 2018 Jul;50:18-30. doi: 10.1016/j.yfrne.2017.09.002. Epub 2017 Sep 30. DOI: https://doi.org/10.1016/j.yfrne.2017.09.002
Wright WG, Gurfinkel VS, King LA, Nutt JG, Cordo PJ, Horak FB. Axial kinesthesia is impaired in Parkinson's disease: effects of levodopa. Exp Neurol. 2010 Sep;225(1):202-9. doi: 10. 1016/j.expneurol.2010.06.016. Epub 2010 Jul 1. DOI: https://doi.org/10.1016/j.expneurol.2010.06.016
Copyright (c) 2022 The Authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/ejtm.2022.10884
https://doi.org/10.4081/ejtm.2022.10884



