The potential effect of leukocyte filtration methods on erythrocyte-derived microvesicles: One step forward
Accepted: 18 July 2022
HTML: 8
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
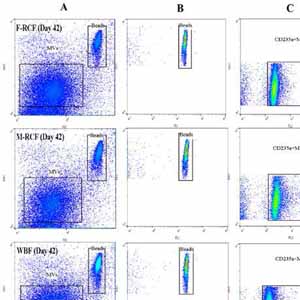
By harmonizing the pre-preparation conditions and also removing some donors’ variations, the current study took one step forward to investigate whether different leukocyte filtration sets influence the quality of RBCs throughout the storage time. Twelve whole blood units were collected, and each unit was split into three equal parts. Thirty-six divided bags were filtered using three different leukocyte-filtration sets including Red Cell and Whole Blood Filters (12 units per filter). The prepared RBCs were refrigerated for up to 42 days and assessed for microvesicle count and size, clotting- and prothrombin time, hemolysis index, and biochemical parameters. A significant increment in erythrocytes microvesicle count (EMVs/μL) was observed during the time in the three filtration sets. The number of EMVs in WBF-RBCs was higher (~1.6 fold) than in F-RCF on day 42 (p=0.035). Interestingly the median fluorescence intensity of EMVs decreased during the storage. The size of MVs rose during the time without any significant differences among the filters. Coagulation time decreased in RBCs over the storage, with no significant differences among the filters. Hemolysis index and lactate concentration increased while glucose level decreased significantly throughout the time. The changes in WBF-RBCs were more drastic rather than RCF-RBCs. The only significant difference in the count of EMVs was between WBF and F-RCF components on day 42. Though the changes in WBF products were more drastic, all the values fell within the standard limits. Accordingly, all three filtration sets can be considered.
Gamonet C, Mourey G, Aupet S, Biichle S, Petitjean R, Vidal C, Pugin A, Naegelen C, Tiberghien P, Morel P, Angelot-Delettre F, Seilles E, Saas P, Bardiaux L, Garnache-Ottou F. How to quantify microparticles in RBCs? A validated flow cytometry method allows the detection of an increase in microparticles during storage. Transfusion. 2017 Mar;57(3):504-516. DOI: https://doi.org/10.1111/trf.13989
Maheen R, Yasir A, Omar N, Nazish M, Navida M, Rao SA, Muhammad R, Shahida M. Levels of red blood cells derived microparticles in stored erythrocyte concentrate. Afr J Pharm Pharmacol. 2020 Jul 31;14(6):185-91. DOI: https://doi.org/10.5897/AJPP2020.5126
Almizraq RJ, Holovati JL, Acker JP. Characteristics of Extracellular Vesicles in Red Blood Concentrates Change with Storage Time and Blood Manufacturing Method. Transfus Med Hemother. 2018 May;45(3):185-193. DOI: https://doi.org/10.1159/000486137
Pandey P, Pande A, Setya D, Kumar P, Shanker A. Comparative Study for Measurement of Residual Leucocytes in Leucodepleted Red Blood Cells by Two Different Methods. Indian J Hematol Blood Transfus. 2020 Oct;36(4):740-744. DOI: https://doi.org/10.1007/s12288-020-01325-5
Almizraq R, Tchir JD, Holovati JL, Acker JP. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion. 2013 Oct;53(10):2258-67. DOI: https://doi.org/10.1111/trf.12080
Delobel J, Barelli S, Canellini G, Prudent M, Lion N, Tissot JD. Red blood cell microvesicles: a storage lesion or a possible salvage mechanism ISBT Sci Ser. 2016 Jan;11(S1):171-7. DOI: https://doi.org/10.1111/voxs.12179
Hashemi Tayer A, Amirizadeh N, Mghsodlu M, Nikogoftar M, Deyhim MR, Ahmadinejad M. Evaluation of blood storage lesions in leuko-depleted red blood cell units. Iranian J Ped Hematol Oncol. 2017 Jul 10;7(3):171-9.
Freitas Leal JK, Lasonder E, Sharma V, Schiller J, Fanelli G, Rinalducci S, Brock R, Bosman G. Vesiculation of Red Blood Cells in the Blood Bank: A Multi-Omics Approach towards Identification of Causes and Consequences. Proteomes. 2020 Mar 31;8(2):6. DOI: https://doi.org/10.3390/proteomes8020006
Kent MW, Kelher MR, West FB, Silliman CC. The pro-inflammatory potential of microparticles in red blood cell units. Transfus Med. 2014 Jun;24(3):176-81. DOI: https://doi.org/10.1111/tme.12123
Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res Int. 2018 Jan 30;2018:8545347. DOI: https://doi.org/10.1155/2018/8545347
Rubin O, Canellini G, Delobel J, Lion N, Tissot JD. Red blood cell microparticles: clinical relevance. Transfus Med Hemother. 2012 Oct;39(5):342-7. Epub 2012 Aug 27. DOI: https://doi.org/10.1159/000342228
Hashemi Tayer A, Amirizadeh N, Ahmadinejad M, Nikougoftar M, Deyhim MR, Zolfaghari S. Procoagulant Activity of Red Blood Cell-Derived Microvesicles during Red Cell Storage. Transfus Med Hemother. 2019 Aug;46(4):224-230. Epub 2018 Nov 13. DOI: https://doi.org/10.1159/000494367
Thangaraju K, Neerukonda SN, Katneni U, Buehler PW. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int J Mol Sci. 2020 Dec 25;22(1):153. DOI: https://doi.org/10.3390/ijms22010153
Almizraq RJ, Norris PJ, Inglis H, Menocha S, Wirtz MR, Juffermans N, Pandey S, Spinella PC, Acker JP, Muszynski JA. Blood manufacturing methods affect red blood cell product characteristics and immunomodulatory activity. Blood Adv. 2018 Sep 25;2(18):2296-2306. DOI: https://doi.org/10.1182/bloodadvances.2018021931
Heddle NM, Arnold DM, Acker JP, Liu Y, Barty RL, Eikelboom JW, Webert KE, Hsia CC, O'Brien SF, Cook RJ. Red blood cell processing methods and in-hospital mortality: a transfusion registry cohort study. Lancet Haematol. 2016 May;3(5):e246-54. DOI: https://doi.org/10.1016/S2352-3026(16)00020-X
Bicalho B, Pereira AS, Acker JP. Buffy coat (top/bottom)- and whole-blood filtration (top/top)-produced red cell concentrates differ in size of extracellular vesicles. Vox Sang. 2015 Oct;109(3):214-20. DOI: https://doi.org/10.1111/vox.12272
Menck K, Bleckmann A, Schulz M, Ries L, Binder C. Isolation and Characterization of Microvesicles from Peripheral Blood. J Vis Exp. 2017 Jan 6;(119):55057. DOI: https://doi.org/10.3791/55057
Ghezelbash B, Azarkeivan A, Pourfathollah AA, Deyhim M, Hajati E, Goodarzi A. Comparative Evaluation of Biochemical and Hematological Parameters of Pre-Storage Leukoreduction during RBC Storage. Int J Hematol Oncol Stem Cell Res. 2018 Jan 1;12(1):35-42.
Sawant RB, Jathar SK, Rajadhyaksha SB, Kadam PT. Red cell hemolysis during processing and storage. Asian J Transfus Sci. 2007 Jul;1(2):47-51. DOI: https://doi.org/10.4103/0973-6247.33446
Almizraq RJ, Seghatchian J, Acker JP. Extracellular vesicles in transfusion-related immunomodulation and the role of blood component manufacturing. Transfus Apher Sci. 2016 Dec;55(3):281-291. DOI: https://doi.org/10.1016/j.transci.2016.10.018
Rubin O, Crettaz D, Canellini G, Tissot JD, Lion N. Microparticles in stored red blood cells: an approach using flow cytometry and proteomic tools. Vox Sang. 2008 Nov;95(4):288-97. DOI: https://doi.org/10.1111/j.1423-0410.2008.01101.x
Rubin O, Crettaz D, Tissot JD, Lion N. Microparticles in stored red blood cells: submicron clotting bombs? Blood Transfus. 2010 Jun;8 Suppl 3(Suppl 3):s31-8.
Gamonet C, Desmarets M, Mourey G, Biichle S, Aupet S, Laheurte C, François A, Resch E, Bigey F, Binda D, Bardiaux L, Naegelen C, Marpaux N, Delettre FA, Saas P, Morel P, Tiberghien P, Lacroix J, Capellier G, Vidal C, Garnache-Ottou F. Processing methods and storage duration impact extracellular vesicle counts in red blood cell units. Blood Adv. 2020 Nov 10;4(21):5527-5539. DOI: https://doi.org/10.1182/bloodadvances.2020001658
Fouda R, Enein AA, El-Desoukey NA, Elfetouh RM, Hafez AM. Impact of storage, leukofiltration, and ascorbic acid fortification on red cell-derived microparticles in stored packed red blood cells: A flow cytometric and procoagulant study. J Appl Hematol. 2020 Apr 1;11(2):51. DOI: https://doi.org/10.4103/joah.joah_76_19
Aung HH, Tung JP, Dean MM, Flower RL, Pecheniuk NM. Procoagulant role of microparticles in routine storage of packed red blood cells: potential risk for prothrombotic post-transfusion complications. Pathology. 2017 Jan;49(1):62-69. DOI: https://doi.org/10.1016/j.pathol.2016.10.001
Bakkour S, Acker JP, Chafets DM, Inglis HC, Norris PJ, Lee TH, Busch MP. Manufacturing method affects mitochondrial DNA release and extracellular vesicle composition in stored red blood cells. Vox Sang. 2016 Jul;111(1):22-32. DOI: https://doi.org/10.1111/vox.12390
Sparrow RL, Healey G, Patton KA, Veale MF. Red blood cell age determines the impact of storage and leukocyte burden on cell adhesion molecules, glycophorin A and the release of annexin V. Transfus Apher Sci. 2006 Feb;34(1):15-23. DOI: https://doi.org/10.1016/j.transci.2005.09.006
Noulsri E, Palasuwan A. Effects of donor age, donor sex, blood-component processing, and storage on cell-derived microparticle concentrations in routine blood-component preparation. Transfus Apher Sci. 2018 Aug;57(4):587-592. DOI: https://doi.org/10.1016/j.transci.2018.07.018
Nemkov T, Skinner SC, Nader E, Stefanoni D, Robert M, Cendali F, Stauffer E, Cibiel A, Boisson C, Connes P, D'Alessandro A. Acute Cycling Exercise Induces Changes in Red Blood Cell Deformability and Membrane Lipid Remodeling. Int J Mol Sci. 2021 Jan 18;22(2):896. DOI: https://doi.org/10.3390/ijms22020896
Noubouossie DF, Henderson MW, Mooberry M, Ilich A, Ellsworth P, Piegore M, Skinner SC, Pawlinski R, Welsby I, Renné T, Hoffman M, Monroe DM, Key NS. Red blood cell microvesicles activate the contact system, leading to factor IX activation via 2 independent pathways. Blood. 2020 Mar 5;135(10):755-765. DOI: https://doi.org/10.1182/blood.2019001643
Stavrou EX. Thromboinflammatory effects of RBC microvesicles. Blood. 2020 Mar 5;135(10):708-709. DOI: https://doi.org/10.1182/blood.2020004985
Grisendi G, Finetti E, Manganaro D, Cordova N, Montagnani G, Spano C, Prapa M, Guarneri V, Otsuru S, Horwitz EM, Mari G, Dominici M. Detection of microparticles from human red blood cells by multiparametric flow cytometry. Blood Transfus. 2015 Apr;13(2):274-80. Epub 2014 Oct 23.
Marabi PM, Musyoki SK, Amayo A. Evaluation of cellular changes in blood stored for transfusion at Bungoma County Referral Hospital, Kenya. Pan Afr Med J. 2021 Mar 17;38:280. DOI: https://doi.org/10.11604/pamj.2021.38.280.22327
Oyet C, Okongo B, Onyuthi RA, Muwanguzi E. Biochemical changes in stored donor units: implications on the efficacy of blood transfusion. J Blood Med. 2018 Jun 25;9:111-115. DOI: https://doi.org/10.2147/JBM.S163651
Verma M, Dahiya K, Malik D, Sehgal PK, Devi R, Soni A, Ghalaut VG. Effect of blood storage on complete biochemistry. J Blood Disord Transfus. 2015;6(6):1-4. DOI: https://doi.org/10.4172/2155-9864.1000329
Dinkla S, Peppelman M, Van Der Raadt J, Atsma F, Novotný VM, Van Kraaij MG, Joosten I, Bosman GJ. Phosphatidylserine exposure on stored red blood cells as a parameter for donor-dependent variation in product quality. Blood Transfus. 2014 Apr;12(2):204-9.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/ejtm.2022.10708
https://doi.org/10.4081/ejtm.2022.10708



