Evaluation of Short Chain Fatty Acids (SCFAs) intestinal absorption, following digestion and fermentation of a novel medical device containing partially-hydrolyzed Guar gum plus simethicone
Accepted: April 1, 2023
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
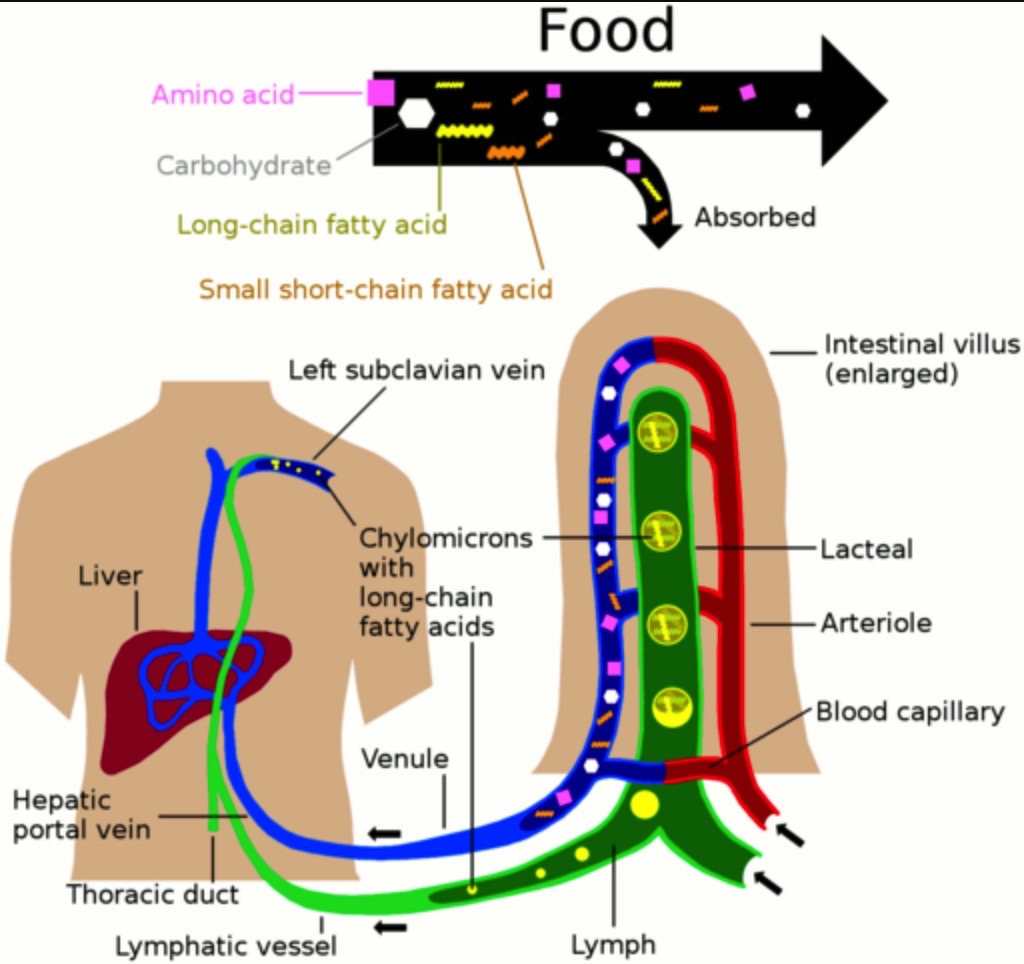
Irritable Bowel Syndrome (IBS) is a common disease characterized by alternate symptoms (diarrhea and constipation) and intestinal gas overproduction. A new medical device (Fibergone®), containing Partially Hydrolyzed Guar Gum (PHGG) and Simethicone (SM) has been proposed for managing patients with bowel disorders. PHGG acts also as a prebiotic so increasing the Short-Chain Fatty Acid (SCFA) production, useful for intestinal physiology. This in vitro study investigated the effects exerted by PHGG+SM on SCFA production and their intestinal absorption following in vitro digestive process and fermentation model. An in vitro model evaluated the digestive process and fermentation using simulated digestive fluids and a human intestinal epithelium in vitro model derived from based on intestinal adenocarcinoma Caco-2 cells (ATCC, HTB-37TM) and organized as a functional monolayer on Transwell® inserts. PHGG+SM was added in experiments and compared with a control (non-treated). SCFA production and absorption were assessed. Viability and barrier integrity of the intestinal epithelium model were also evaluated. PHGG+SM significantly (p<0.05) increased SCFAs content after fermentation, indicating that this medical device is effectively fermented at the large intestine level. However, in relation to SCFAs bioavailability, their absorption did not increase compared to the non-treated condition in the light of the physiological contribution of SCFAs resulting from the microflora. PHGG+SM did not affect intestinal epithelium apparent permeability (Papp) and viability. This in vitro study documented that partially hydrolyzed guar gum combined with simethicone significantly affects short-chain fatty acids production and consequently could be fruitfully employed in managing patients with intestinal disorders.
Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome a clinical review. JAMA 2015;313:949-8 DOI: https://doi.org/10.1001/jama.2015.0954
Fond G, Loundou A, Hamdani N. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 2014;264:651-60. DOI: https://doi.org/10.1007/s00406-014-0502-z
Fairbrass KM, Costantino SJ, Gracie D, Ford AC. Prevalence of irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease in remission: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020;5:1053-62. DOI: https://doi.org/10.1016/S2468-1253(20)30300-9
Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016;S0016-5085(16)00222-5.
Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet Gastroenterol 2020;5:1053-62. DOI: https://doi.org/10.1016/S0140-6736(20)31548-8
Defrees DN, Bailey J. Irritable bowel syndrome epidemiology, pathophysiology, diagnosis, and treatment. Prim Care Clin Office 2017;44:655-71. DOI: https://doi.org/10.1016/j.pop.2017.07.009
Adriani A, Ribaldone DG, Astegiano M, et al. Irritablebowelsyndrome: the clinical approach. Panminerva Medica 2018;60:213-22. DOI: https://doi.org/10.23736/S0031-0808.18.03541-3
Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: management of irritable bowel syndrome. Am J Gastroenterol 2021;116:17-44. DOI: https://doi.org/10.14309/ajg.0000000000001036
Yoon SJ, Chu DC, Raj Juneja L. Chemical and physical properties, safety and application of partially hydrolized guar gum as dietary fiber. J Clin Biochem Nutr 2008;42:1‑7. DOI: https://doi.org/10.3164/jcbn.2008001
Giannini EG, Mansi C, Dulbecco P, Savarino V. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition 2006;22:334-42. DOI: https://doi.org/10.1016/j.nut.2005.10.003
Mazzawi T. Gut microbiota manipulation in irritable bowel syndrome. Microorganisms 2022;10:1332.
Moolla M, Dang JT, Shaw A, et al. Simethicone decreases bloating and improves bowel preparation effectiveness: a systematic review and meta-analysis. Surg Endosc 2019;33:3899-909. DOI: https://doi.org/10.1007/s00464-019-07066-5
Minekus M, Alminger M, Alvito P, et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct 2014;5:1113-24. DOI: https://doi.org/10.1039/C3FO60702J
Carlson JL, Erickson JM, Hess JM, et al. Prebiotic dietary fiber and gut health: comparing the in vitro fermentations of beta-glucan, inulin and xylo-oligosaccharide. Nutrients 2017;9:1361. DOI: https://doi.org/10.3390/nu9121361
US National Institute of Standards and Technology. NIST Standard Reference Database 1A, 2008. Available from: https://www.nist.gov/system/files/documents/srd/NIST1aVer22Man.pdf
Kabakov AE, Gabai VL. Cell death and survival assays. Methods Mol Biol 2018;1709:107-27. DOI: https://doi.org/10.1007/978-1-4939-7477-1_9
Yan H, Ajuwon KM. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS One 2017;12:e0179586. DOI: https://doi.org/10.1371/journal.pone.0179586
Schmulson MJ, Chiu-Ugalde J, Saez-Rios A, et al. Efficacy of the combination of pinaverium bromide 100 mg plus simethicone 300 mg in abdominal pain and bloating in irritable bowel syndrome: a randomized, placebo-controlled Trial. J Clin Gastroenterol 2020;54:e30-9. DOI: https://doi.org/10.1097/MCG.0000000000001242
Paul SP, Barnard P, Edate S, Candy DC. Stool consistency and abdominal pain in irritable bowel syndrome may be improved by partially hydrolysed guar gum. J Pediatr Gastroenterol Nutr 2011;53:582-3. DOI: https://doi.org/10.1097/MPG.0b013e3182307c7a
Russo L, Andreozzi P, Zito FP, et al. Partially hydrolyzed guar gum in the treatment of irritable bowel syndrome with constipation: effects of gender, age, and body mass index. Saudi J Gastroenterol 2015;21:104-10. DOI: https://doi.org/10.4103/1319-3767.153835
Niv E, Halak A, Tiommy E, et al. Randomized clinical study: Partially hydrolyzed guar gum (PHGG) versus placebo in the treatment of patients with irritable bowel syndrome. Nutr Metab (Lond) 2016;13:10. DOI: https://doi.org/10.1186/s12986-016-0070-5
Mazzawi T. Gut microbiota manipulation in Irritable Bowel Syndrome. Microorganisms 2022;10:133. DOI: https://doi.org/10.3390/microorganisms10071332
Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time? Nutr Clin Pract 2006;21:351-66. DOI: https://doi.org/10.1177/0115426506021004351
Ciprandi G. Partially-hydrolyzed guar gum plus simethicone in patients with irritable bowel syndrome: a real-life survey. Gazzetta Med Ital 2023 (in press). DOI: https://doi.org/10.23736/S0393-3660.23.05037-4
Aragona SE, Baldini E, Bonsignore N, et al. Guar fibers and simethicone: a winning synergy for the treatment of IBS: an observational comparative study of irritable bowel symptoms. Gazzetta Med Ital 2023 (in press). DOI: https://doi.org/10.23736/S0393-3660.23.05044-1
Copyright (c) 2023 the Author(s)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/jbr.2023.11154
https://doi.org/10.4081/jbr.2023.11154



