Molecular docking analysis of seagrass (Enhalus acoroides) phytochemical compounds as an antidiabetic
Accepted: January 27, 2022
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
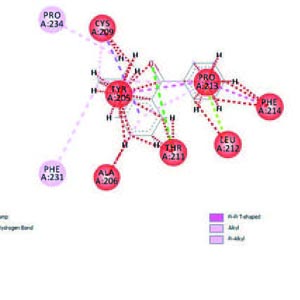
Enhalus acoroides have potential to inhibit the α-glucosidase enzyme and as an antidiabetic drug. Twenty-seven phytochemical compounds of seagrass (E. acoroides) were analyzed by molecular docking method. All possible candidate compounds predicted ADME pharmacokinetic properties using the swiss ADME website. A molecular docking analysis was carried out using the PyRx 0.8 Autodock Vina software. Furthermore, the interaction analysis between molecules was carried out using PyMOL software and the Discovery Studio Visualizer BIOVIA. There were 17 of the 27 compounds which had the best potency as oral antidiabetic drug candidates. The validation results showed that all ligands had aroot mean score deviation (RMSD) value <2Å with the best value of 0.0. The binding affinity with the strongest bond value was -9.2 (kcal/mol) on the NAMPT bond with tannin, while the weakest value was 40.01 at 3l4y (α-glucosidase) with 3-methyl. The 2h6d receptor can bind to all ligands, and the α-glucosidase receptor can bind to two test ligands. The docking method used in this study was valid, and the phytochemical compounds of seagrass have the potential to be an alternative to antidiabetic drugs.
Akrom, Harjanti PD, Armansyah. Hypoglycemic effects of ethanol extract of sweet cassava (Ipomoea batatas P) (Eeukr) in swiss alloxan-induced mice. Pharm J 2014;4:65-76. DOI: https://doi.org/10.12928/pharmaciana.v4i1.400
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018;14:88-98. DOI: https://doi.org/10.1038/nrendo.2017.151
Wresdiyati T, Sa’diah S, Winarto A, Febriyani V. Alpha-glucosidase inhibition and hypoglycemic activities of Sweitenia mahagoni seed extract. HAYATI J Biosci 2015;22:73-8. DOI: https://doi.org/10.4308/hjb.22.2.73
Rina N, Antarsih. Efek etil asetat daun lamun (Enhalus acoroides (LF) Royle) terhadap kadar MDA dan GSH mencit jantan tua). J Pen Kes 2019;4:2-56. DOI: https://doi.org/10.22435/sel.v4i2.1449
Bare Y, Maulidi A, Sari DRT, Tiring SSND. In silico study predicts the potential of 6-gingerol as an inhibitor of c-jun n-terminal kinases (JNK). Mat SciNet J 2019;1:59-63. DOI: https://doi.org/10.36873/jjms.v1i2.211
Colquitt RB, Colquhoun DA, Thiele RH. In silico modelling as physiologic system. Best Pract Res Cl An 2011;25:499-510. DOI: https://doi.org/10.1016/j.bpa.2011.08.006
Amudha P, Vanitha V. Toxicological, biochemical and histopathological evaluation of the ethanolic extract of seagrass-Enhalus acoroides in albino wistar rats. Prog Biotechnol 2019;19:1-10. DOI: https://doi.org/10.1016/j.bcab.2019.101082
Rahakbauw ID, Watuguly T. Analysis of Enhalus acoroides seagrass leaves compound in the coastal waters of Waai Village, Central Maluku Regency. Biopendix 2016;3:1-53. DOI: https://doi.org/10.30598/biopendixvol3issue1page53-62
Ganesh J. Faunal associates, trace metal content and bioefficacy of seagrass Enhalus acoroides (LF) Royle, 1839 and Halodule uninervis (Forsk.) Asch. 1882 from Kattumavadi, Palk Bay, Tamil Nadu, India. Geo 2011.
Yuniarto A, Selifiana N. Aktivitas inhibisi enzim alfa-glukosidase dari ekstrak rimpang bangle (Zingiber cassumunar Roxb.) secara in vitro. Med Pharm Ind 2018;2:1-22. DOI: https://doi.org/10.24123/mpi.v2i1.1299
Menajang FSI, Mahmudi M, Yanuhar U, Herawati EY. Evaluation of phytochemical and superoxide dismutase activities of Enhalus acoroides (Lf) Royle from the coastal waters of North Sulawesi, Indonesia. Vet World 2020;13:676-80. DOI: https://doi.org/10.14202/vetworld.2020.676-680
Zuhro F, Puspitasari E, Muslichah S, Hidayat MA. Inhibitor α-Glukosidase ekstrak etanol daun kenitu (Chrysophyllum cainito L.). Pus Kes 2016;4:1-7.
Wati W, Widodo GP, Herowati R. Prediction of pharmacokinetics parameter and molecular docking study of antidiabetic compounds from Syzogium polyanthum and Syzygium cumini. J Sci Appl Chem 2020;23:189-95. DOI: https://doi.org/10.14710/jksa.23.6.189-195
Rajan K, Zielensy A, Steinbeck C. STOUT: SMILES to IUPAC names using neural machine translation. Chem J 2021;13:1-14. DOI: https://doi.org/10.1186/s13321-021-00512-4
Patil R, Das S, Stanley A, Yadav L. Optimized hydrophobic interactions and hydrogen bonding at the target-ligand interface leads the pathways of drug-designing. PLoS One 2010;5:12-5. DOI: https://doi.org/10.1371/journal.pone.0012029
Rathi P, Ludlow F, Verdonk M. Practical high-quality electrostatic potential surfaces for drug discovery using a graph-convolutional deep neural network. Med Chem J 2019;63:8778-90. DOI: https://doi.org/10.1021/acs.jmedchem.9b01129
Frimayanti N, Zamri A, Eryanti Y, et al. Docking and molecular dynamic simulations study to search curcumin analogue compounds as potential inhibitor gainst SARS-C0V-2: a computational approach. J Sci Appl Chem 2021;3:85-90. DOI: https://doi.org/10.14710/jksa.24.3.85-90
Rachmania RA. Validation of virtual screening protocols and analysis of natural-based cancer cell antiproliferation inhibitor interactions against cyclin-dependent kinase 4 (CDK4) receptors. Med Pharm J 2019;16:21-40. DOI: https://doi.org/10.12928/mf.v16i1.12101
Ibrahim MA, Bester MJ, Neitz AW, Gaspar ARM. Rational in silico design of novel α-glucosidase inhibitory peptides and in vitro evaluation of promising candidates. Biomed Pharmacoter J 2018;107:234-42. DOI: https://doi.org/10.1016/j.biopha.2018.07.163
Medina, KD, YS Moreno, D. Milenkovic, et al. In silico analysis of antidiabetic potential of phenolic compounds from blue corn (Zea mays L.) and black bean (Phaseolus vulgaris L.). Heliyon J 2020;6:1-3. DOI: https://doi.org/10.1016/j.heliyon.2020.e03632
Morais FS, Canuto KM, Riberio PR, et al. Chemical profiling of secondary metabolites from Himatanthus drasticus (Mart.) Plumel latex with inhibitory action against the enzymes α-amylase and α-glucosidase: In vitro and in silico assays. Ethnopharmacologie 2020;253:1-9. DOI: https://doi.org/10.1016/j.jep.2020.112644
Vinsiah R, Fadhillah. Study of hydrogen bonding in the methanol-methanol and ethanol-ethanol system using the dynamic molecular method. Mat Nat Science J 2018;15:1-14.
Ali M, Khan KM, Mahdavi M, et al. Synthesis, in vitro and in silico screening ff 2-amino-4-aryl-6-(phenylthio) pyridine-3,5-dicarbonitriles as novel a-glucosidase inhibitors. Bioorg Chem 2020;100:1-13. DOI: https://doi.org/10.1016/j.bioorg.2020.103879
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/jbr.2022.10224
https://doi.org/10.4081/jbr.2022.10224



