The location of InsP3 receptors in Purkinje cells of murine cerebellum does not supports a direct interaction in the transfer of calcium ions between ER and mitochondria
Accepted: 6 August 2021
HTML: 25
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
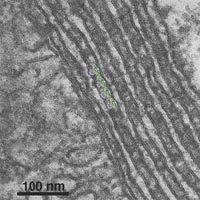
The inositol-3-phosphate receptors (IP3Rs) of cerebellar Purkinje cells are located in abundant, large stacks of endoplasmic reticulum (ER) cisternae. Using thin section electron microscopy, we identify very frequent associations of the ER stacks with mitochondria. The associations have two components: a single, close ER-mitochondria contact on one side to the stack, and multiple layers of ER cisternae decorated by IP3Rs receptors on the side away from the mitochondria. Due to their location in the stacks, IP3Rs are never in contact with the mitochondria, although they are in their vicinity. We conclude that transfer of Ca2+ between ER and mitochondria is not directly mediated by IP3Rs, but is based on mitochondrial Ca2+ uptake from the local cytoplasmic spikes during IP3Rs’ activity.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejtm.2021.9935
https://doi.org/10.4081/ejtm.2021.9935



