A case report of severe veno-occlusive disease following autologous stem cell transplantation successfully treated with Defibrotide
Accepted: 18 June 2020
HTML: 29
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
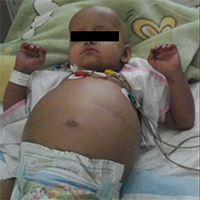
Veno-occlusive disease (VOD) is one of the complications of hematopoietic stem cell transplantation that can also be caused by high-dose chemotherapy. This complication can lead to high mortality following bone marrow transplantation. It is more common after allogeneic stem cell transplantation, and is rare after autologous stem cell transplantation. While mild cases of VOD may reduce over a period of a few weeks, very severe cases can cause multi-organ damage, which has a high mortality. is therefore required with early diagnosis and treatment of this complication. In this paper, we present a sever VOD case after autologous stem cell transplantation, that was treated successfully with Defibrotide. The patient was a 14-month-old girl who has neuroblastoma with bone metastasis. VOD should be considered in the differential diagnosis of haematopoietic stem cell transplantation recipients who present with unexplained liver injuries, ascites and/or multi organ failure. Recipients of haematopoeitic stem cell transplantation who present with unexplained liver injuries, ascites and/or multi organ failure should have VOD considered in their differential diagnosis. If there is severe VOD diagnosed, then Defibrotide could be an option for treatment.
Richardson P, Aggarwal S, Topaloglu O, et al. Systematic review of defibrotide studies in the treatment of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS). Bone Marrow Transplant 2019;54;1951–62 doi: 10.1038/s41409-019 0474-8.
Corbacioglu S, Carreras E, Ansari M, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2018; 53:138–45. DOI: https://doi.org/10.1038/bmt.2017.161
Mohty M, Malard F, Abecassis M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015; 50:781–9. DOI: https://doi.org/10.1038/bmt.2015.52
Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 2011; 46:1495–502. DOI: https://doi.org/10.1038/bmt.2011.65
Mohty M, Malard F, Abecassis M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016; 51:906–12. DOI: https://doi.org/10.1038/bmt.2016.130
Castellino A, Guidi S, Dellacasa CM, et al. Late-Onset Hepatic Veno-Occlusive Disease after Allografting: Report of Two Cases with Atypical Clinical Features Successfully Treated with Defibrotide. Mediterr J Hematol Infect Dis. 2018; 10: e2018001. DOI: https://doi.org/10.4084/mjhid.2018.001
Palomo M, Mir E, Rovira M, et al. What is going on between defibrotide and endothelial cells? Snapshots reveal the hot spots of their romance. Blood. 2016; 127:1719–27. DOI: https://doi.org/10.1182/blood-2015-10-676114
Strouse C, Richardson P, Prentice G, et al. Defibrotide for treatment of severe veno-occlusive disease in pediatrics and adults: an exploratory analysis using data from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2016; 22:1306–12.
Richardson PG, Riches ML, Kernan NA, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016; 127:1656–65. DOI: https://doi.org/10.1182/blood-2015-10-676924
Corbacioglu S, Cesaro S, Faraci M, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomized controlled trial. The Lancet. 2012; 379:1301–9. DOI: https://doi.org/10.1016/S0140-6736(11)61938-7
Strouse C, Richardson P, Prentice G, et al. Defibrotide for Treatment of Severe Veno-Occlusive Disease in Pediatrics and Adults: An Exploratory Analysis Using Data from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2016; 22:1306–12. DOI: https://doi.org/10.1016/j.bbmt.2016.04.011
Richardson PG, Elias AD, Krishnan A, et al. Treatment of severe veno-occlusive disease with defibrotide: compassionate use results in response without significant toxicity in a high-risk population. Blood. 1998; 92:737–744.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/ejtm.2020.9161
https://doi.org/10.4081/ejtm.2020.9161



