Inhibition of Activin/Myostatin signalling induces skeletal muscle hypertrophy but impairs mouse testicular development
HTML: 25
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
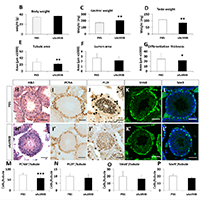
Numerous approaches are being developed to promote post-natal muscle growth based on attenuating Myostatin/Activin signalling for clinical uses such as the treatment neuromuscular diseases, cancer cachexia and sarcopenia. However there have been concerns about the effects of inhibiting Activin on tissues other than skeletal muscle. We intraperitoneally injected mice with the Activin ligand trap, sActRIIB, in young, adult and a progeric mouse model. Treatment at any stage in the life of the mouse rapidly increased muscle mass. However at all stages of life the treatment decreased the weights of the testis. Not only were the testis smaller, but they contained fewer sperm compared to untreated mice. We found that the hypertrophic muscle phenotype was lost after the cessation of sActRIIB treatment but abnormal testis phenotype persisted. In summary, attenuation of Myostatin/Activin signalling inhibited testis development. Future use of molecules based on a similar mode of action to promote muscle growth should be carefully profiled for adverse side-effects on the testis. However the effectiveness of sActRIIB as a modulator of Activin function provides a possible therapeutic strategy to alleviate testicular seminoma development.
Matsakas A, Patel K. Skeletal muscle fibre plasticity in response to selected environmental and physiological stimuli. Histol Histopathol. 2009;24(5):611-29.
Waterlow JC, Jackson AA. Nutrition and protein turnover in man. Br Med Bull. 1981;37(1):5-10. DOI: https://doi.org/10.1093/oxfordjournals.bmb.a071676
Poortmans JR, Carpentier A, Pereira-Lancha LO, Lancha A, Jr. Protein turnover, amino acid requirements and recommendations for athletes and active populations. Braz J Med Biol Res. 2012;45(10):875-90. DOI: https://doi.org/10.1590/S0100-879X2012007500096
Sjoqvist F, Garle M, Rane A. Use of doping agents, particularly anabolic steroids, in sports and society. Lancet. 2008;371(9627):1872-82. DOI: https://doi.org/10.1016/S0140-6736(08)60801-6
Windisch PA, Papatheofanis FJ, Matuszewski KA. Recombinant human growth hormone for AIDS-associated wasting. Ann Pharmacother. 1998;32(4):437-45. DOI: https://doi.org/10.1345/aph.17255
Borst SE, Lowenthal DT. Role of IGF-I in muscular atrophy of aging. Endocrine. 1997;7(1):61-3. DOI: https://doi.org/10.1007/BF02778065
McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83-90. DOI: https://doi.org/10.1038/387083a0
Aiello D, Patel K, Lasagna E. The myostatin gene: an overview of mechanisms of action and its relevance to livestock animals. Anim Genet. 2018;49(6):505-19. DOI: https://doi.org/10.1111/age.12696
Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98(16):9306-11. DOI: https://doi.org/10.1073/pnas.151270098
Hill JJ, Qiu Y, Hewick RM, Wolfman NM. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: a novel protein with protease inhibitor and follistatin domains. Mol Endocrinol. 2003;17(6):1144-54. DOI: https://doi.org/10.1210/me.2002-0366
Anderson SB, Goldberg AL, Whitman M. Identification of a novel pool of extracellular pro-myostatin in skeletal muscle. J Biol Chem. 2008;283(11):7027-35. DOI: https://doi.org/10.1074/jbc.M706678200
Matsakas A, Foster K, Otto A, Macharia R, Elashry MI, Feist S, et al. Molecular, cellular and physiological investigation of myostatin propeptide-mediated muscle growth in adult mice. Neuromuscul Disord. 2009;19(7):489-99. DOI: https://doi.org/10.1016/j.nmd.2009.06.367
Olsen OE, Wader KF, Hella H, Mylin AK, Turesson I, Nesthus I, et al. Activin A inhibits BMP-signaling by binding ACVR2A and ACVR2B. Cell Commun Signal. 2015;13:27. DOI: https://doi.org/10.1186/s12964-015-0104-z
Lach-Trifilieff E, Minetti GC, Sheppard K, Ibebunjo C, Feige JN, Hartmann S, et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol Cell Biol. 2014;34(4):606-18. DOI: https://doi.org/10.1128/MCB.01307-13
Hoogaars WM, Mouisel E, Pasternack A, Hulmi JJ, Relizani K, Schuelke M, et al. Combined effect of AAV-U7-induced dystrophin exon skipping and soluble activin Type IIB receptor in mdx mice. Hum Gene Ther. 2012;23(12):1269-79. DOI: https://doi.org/10.1089/hum.2012.056
Lee SJ, Reed LA, Davies MV, Girgenrath S, Goad ME, Tomkinson KN, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci U S A. 2005;102(50):18117-22. DOI: https://doi.org/10.1073/pnas.0505996102
Sartori R, Schirwis E, Blaauw B, Bortolanza S, Zhao J, Enzo E, et al. BMP signaling controls muscle mass. Nat Genet. 2013;45(11):1309-18. DOI: https://doi.org/10.1038/ng.2772
Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14(1):58-74. DOI: https://doi.org/10.1038/nrd4467
Koncarevic A, Kajimura S, Cornwall-Brady M, Andreucci A, Pullen A, Sako D, et al. A novel therapeutic approach to treating obesity through modulation of TGFbeta signaling. Endocrinology. 2012;153(7):3133-46. DOI: https://doi.org/10.1210/en.2012-1016
Liu H, Zhang R, Chen D, Oyajobi BO, Zhao M. Functional redundancy of type II BMP receptor and type IIB activin receptor in BMP2-induced osteoblast differentiation. J Cell Physiol. 2012;227(3):952-63. DOI: https://doi.org/10.1002/jcp.22802
Latres E, Mastaitis J, Fury W, Miloscio L, Trejos J, Pangilinan J, et al. Activin A more prominently regulates muscle mass in primates than does GDF8. Nat Commun. 2017;8:15153. DOI: https://doi.org/10.1038/ncomms15153
Boitani C, Stefanini M, Fragale A, Morena AR. Activin stimulates Sertoli cell proliferation in a defined period of rat testis development. Endocrinology. 1995;136(12):5438-44. DOI: https://doi.org/10.1210/endo.136.12.7588293
Buzzard JJ, Farnworth PG, De Kretser DM, O'Connor AE, Wreford NG, Morrison JR. Proliferative phase sertoli cells display a developmentally regulated response to activin in vitro. Endocrinology. 2003;144(2):474-83. DOI: https://doi.org/10.1210/en.2002-220595
Buzzard JJ, Loveland KL, O'Bryan MK, O'Connor AE, Bakker M, Hayashi T, et al. Changes in circulating and testicular levels of inhibin A and B and activin A during postnatal development in the rat. Endocrinology. 2004;145(7):3532-41. DOI: https://doi.org/10.1210/en.2003-1036
Kumar TR, Varani S, Wreford NG, Telfer NM, de Kretser DM, Matzuk MM. Male reproductive phenotypes in double mutant mice lacking both FSHbeta and activin receptor IIA. Endocrinology. 2001;142(8):3512-8. DOI: https://doi.org/10.1210/endo.142.8.8336
del Re E, Sidis Y, Fabrizio DA, Lin HY, Schneyer A. Reconstitution and analysis of soluble inhibin and activin receptor complexes in a cell-free system. J Biol Chem. 2004;279(51):53126-35. DOI: https://doi.org/10.1074/jbc.M408090200
Alyodawi K, Vermeij WP, Omairi S, Kretz O, Hopkinson M, Solagna F, et al. Compression of morbidity in a progeroid mouse model through the attenuation of myostatin/activin signalling. J Cachexia Sarcopenia Muscle. 2019. DOI: https://doi.org/10.1002/jcsm.12404
Relizani K, Mouisel E, Giannesini B, Hourde C, Patel K, Morales Gonzalez S, et al. Blockade of ActRIIB signaling triggers muscle fatigability and metabolic myopathy. Mol Ther. 2014;22(8):1423-33. DOI: https://doi.org/10.1038/mt.2014.90
Omairi S, Matsakas A, Degens H, Kretz O, Hansson KA, Solbra AV, et al. Enhanced exercise and regenerative capacity in a mouse model that violates size constraints of oxidative muscle fibres. Elife. 2016;5. DOI: https://doi.org/10.7554/eLife.16940
Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36(6):653-9. DOI: https://doi.org/10.1038/ng1367
Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, et al. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod. 2008;79(1):35-42. DOI: https://doi.org/10.1095/biolreprod.107.066795
Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14(1):62-8. DOI: https://doi.org/10.1038/ng0996-62
Chen Q, Peng H, Lei L, Zhang Y, Kuang H, Cao Y, et al. Aquaporin3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration. Cell Res. 2011;21(6):922-33. DOI: https://doi.org/10.1038/cr.2010.169
Vermeij WP, Dolle ME, Reiling E, Jaarsma D, Payan-Gomez C, Bombardieri CR, et al. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016;537(7620):427-31. DOI: https://doi.org/10.1038/nature19329
Oldknow KJ, Seebacher J, Goswami T, Villen J, Pitsillides AA, O'Shaughnessy PJ, et al. Follistatin-like 3 (FSTL3) mediated silencing of transforming growth factor beta (TGFbeta) signaling is essential for testicular aging and regulating testis size. Endocrinology. 2013;154(3):1310-20. DOI: https://doi.org/10.1210/en.2012-1886
Auharek SA, de Franca LR. Postnatal testis development, Sertoli cell proliferation and number of different spermatogonial types in C57BL/6J mice made transiently hypo- and hyperthyroidic during the neonatal period. J Anat. 2010;216(5):577-88. DOI: https://doi.org/10.1111/j.1469-7580.2010.01219.x
Campbell C, McMillan HJ, Mah JK, Tarnopolsky M, Selby K, McClure T, et al. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: Results of a randomized, placebo-controlled clinical trial. Muscle Nerve. 2017;55(4):458-64. DOI: https://doi.org/10.1002/mus.25268
Shieh PB. Emerging Strategies in the Treatment of Duchenne Muscular Dystrophy. Neurotherapeutics. 2018;15(4):840-8. DOI: https://doi.org/10.1007/s13311-018-00687-z
Roh JD, Hobson R, Chaudhari V, Quintero P, Yeri A, Benson M, et al. Activin type II receptor signaling in cardiac aging and heart failure. Sci Transl Med. 2019;11(482). DOI: https://doi.org/10.1126/scitranslmed.aau8680
Ioannis M, Foivos P, Dimitrios K. A review on the treatment of sporadic inclusion body myositis with Bimagrumab and Alemtuzumab. Int J Neurosci. 2019;129(3):297-302. DOI: https://doi.org/10.1080/00207454.2018.1527329
Harish P, Malerba A, Lu-Nguyen N, Forrest L, Cappellari O, Roth F, et al. Inhibition of myostatin improves muscle atrophy in oculopharyngeal muscular dystrophy (OPMD). J Cachexia Sarcopenia Muscle. 2019. DOI: https://doi.org/10.1002/jcsm.12438
Consitt LA, Clark BC. The Vicious Cycle of Myostatin Signaling in Sarcopenic Obesity: Myostatin Role in Skeletal Muscle Growth, Insulin Signaling and Implications for Clinical Trials. J Frailty Aging. 2018;7(1):21-7.
Tillet E, Bailly S. Emerging roles of BMP9 and BMP10 in hereditary hemorrhagic telangiectasia. Front Genet. 2014;5:456.
Glasser CE, Gartner MR, Wilson D, Miller B, Sherman ML, Attie KM. Locally acting ACE-083 increases muscle volume in healthy volunteers. Muscle Nerve. 2018;57(6):921-6. DOI: https://doi.org/10.1002/mus.26113
Dias V, Meachem S, Rajpert-De Meyts E, McLachlan R, Manuelpillai U, Loveland KL. Activin receptor subunits in normal and dysfunctional adult human testis. Hum Reprod. 2008;23(2):412-20. DOI: https://doi.org/10.1093/humrep/dem343
Morvan F, Rondeau JM, Zou C, Minetti G, Scheufler C, Scharenberg M, et al. Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy. Proc Natl Acad Sci U S A. 2017;114(47):12448-53. DOI: https://doi.org/10.1073/pnas.1707925114
Rooks D, Praestgaard J, Hariry S, Laurent D, Petricoul O, Perry RG, et al. Treatment of Sarcopenia with Bimagrumab: Results from a Phase II, Randomized, Controlled, Proof-of-Concept Study. J Am Geriatr Soc. 2017;65(9):1988-95. DOI: https://doi.org/10.1111/jgs.14927
Garito T, Roubenoff R, Hompesch M, Morrow L, Gomez K, Rooks D, et al. Bimagrumab improves body composition and insulin sensitivity in insulin-resistant individuals. Diabetes Obes Metab. 2018;20(1):94-102. DOI: https://doi.org/10.1111/dom.13042
Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve. 2009;39(3):283-96. DOI: https://doi.org/10.1002/mus.21244
Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, et al. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology. 2004;145(1):318-29. DOI: https://doi.org/10.1210/en.2003-1055
Mithraprabhu S, Mendis S, Meachem SJ, Tubino L, Matzuk MM, Brown CW, et al. Activin bioactivity affects germ cell differentiation in the postnatal mouse testis in vivo. Biol Reprod. 2010;82(5):980-90. DOI: https://doi.org/10.1095/biolreprod.109.079855
Istasse L, Van Eenaeme C, Evrard P, Gabriel A, Baldwin P, Maghuin-Rogister G, et al. Animal performance, plasma hormones and metabolites in Holstein and Belgian Blue growing-fattening bulls. J Anim Sci. 1990;68(9):2666-73. DOI: https://doi.org/10.2527/1990.6892666x
Horwitz H, Andersen JT, Dalhoff KP. Health consequences of androgenic anabolic steroid use. J Intern Med. 2019;285(3):333-40. DOI: https://doi.org/10.1111/joim.12850
Knuth UA, Maniera H, Nieschlag E. Anabolic steroids and semen parameters in bodybuilders. Fertil Steril. 1989;52(6):1041-7. DOI: https://doi.org/10.1016/S0015-0282(16)53172-0
Sell A, Lukazsweski AW, Townsley M. Cues of upper body strength account for most of the variance in men's bodily attractiveness. Proc Biol Sci. 2017;284(1869). DOI: https://doi.org/10.1098/rspb.2017.1819
Mossman JA, Pacey AA. The fertility fitness paradox of anabolic-androgenic steroid abuse in men. J Intern Med. 2019. DOI: https://doi.org/10.1111/joim.12884
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/ejtm.2019.8737
https://doi.org/10.4081/ejtm.2019.8737



