Wound healing effects of Persian walnut (Juglans regia L.) green husk on the incision wound model in rats
Accepted: 22 December 2019
HTML: 23
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
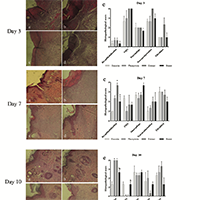
Walnut green husk (WGH) has been mentioned as a wound-healing agent in traditional Iranian medicine. Although previous studies indicated that WGH is a good source of pharmaceutical ingredients, they did not assess its wound healing activity; so the present study set out the scientific validation of the wound healing potential of the Persian walnut. Total phenolic content, reducing power, DPPH, and nitric oxide scavenging activity of aqueous ethanol extract of WGH was evaluated. Forty-eight male Wistar albino rats were divided into four groups of 12 each. An incision wound was created on the dorsal region of each rat. WGH extract (20% w/w), WGH burnt residues (20% w/w), Eucerin, and Phenytoin ointments were used in each group. Wound length, contraction percentage, and histopathological evaluations were recorded on days 3, 7, 10, and 14. Total phenolic content and EC50 values of reducing power, DPPH and nitric oxide scavenging activity of the WGH extract were 61.34 ± 0.64 mg/g dry extract, 0.95 ± 0.02 mg/mL, 0.35 ± 0.01 mg/mL, and 0.28 ± 0.01 mg/mL, respectively. Treated animals with WGH extract showed significantly (p ≤ 0.05) better results for physical and pathological parameters compared to the control group; overall, WGH extract showed better results than WGH burnt residues. The present study indicated that the WGH aqueous ethanol extract has a promising potential for wound healing in the animal model and could be a valuable resource for developing new wound-healing medicines for humans.
Gonzalez AC de O, Costa TF, Andrade Z de A, Medrado ARAP. Wound healing - A literature review. An Bras Dermatol 2016;91:614-20. DOI: https://doi.org/10.1590/abd1806-4841.20164741
Nicolaus C, Junghanns S, Hartmann A, et al. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J Ethnopharmacol 2017;196:94-103. DOI: https://doi.org/10.1016/j.jep.2016.12.006
Nordensram B. Rechinger, K. H. (ed.), Flora Iranica, Fasc. 111–162 (1975–1987). Nord J Bot 1989;8:625-6.
Barreto R, Albuquerque-Júnior R, Araújo A, et al. A Systematic Review of the Wound-Healing Effects of Monoterpenes and Iridoid Derivatives. Molecules 2014;19:846-62. DOI: https://doi.org/10.3390/molecules19010846
Panth N, Paudel KR, Karki R. Phytochemical profile and biological activity of Juglans regia. J Integr Med 2016;14:359-73. DOI: https://doi.org/10.1016/S2095-4964(16)60274-1
Ghasemi K, Ghasemi Y, Ehteshamnia A, et al. Influence of environmental factors on antioxidant activity, phenol and flavonoids contents of walnut (Juglans regia L.) green husks. J Med Plants Res 2011;5:1128-33.
Chew KK, Khoo MZ, Ng SY, et al. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int Food Res J 2011;18:1427.
AĞ ŞELECİ D, GÜMÜŞ ZP, YAVUZ M, et al. A case study on in vitro investigations of the potent biological activities of wheat germ and black cumin seed oil. Turkish J Chem 2015;39:801-12. DOI: https://doi.org/10.3906/kim-1502-72
Berker KI, Güçlü K, Tor İ, Apak R. Comparative evaluation of Fe (III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta 2007;72:1157-65. DOI: https://doi.org/10.1016/j.talanta.2007.01.019
Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull 1988;36:2090-7. DOI: https://doi.org/10.1248/cpb.36.2090
Sousa A, Ferreira I, Barros L, et al. Antioxidant potential of traditional stoned table olives ‘‘Alcaparras”: influence of the solvent and temperature extraction conditions. LWT–Food Sci Technol 2008;41:739-45. DOI: https://doi.org/10.1016/j.lwt.2007.04.003
Ozay Y, Kasim MC, Guzel-Ozay S, et al. Effects of Equisetum arvense Ointment on Diabetic Wound Healing in Rats. Wounds a Compend Clin Res Pract 2013;25:234-41.
Sirak A, Abie G. Manual of standard operating procedure (SOP) for tissue processing. Natl Anim Heal Investig Cent (NAHDIC), Ethiop 2009:8-32.
Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 2009;37:1528-42. DOI: https://doi.org/10.1177/147323000903700531
Tümen İ, Akkol EK, Taştan H, et al. Research on the antioxidant, wound healing, and anti-inflammatory activities and the phytochemical composition of maritime pine ( Pinus pinaster Ait). J Ethnopharmacol 2018;211:235-46. DOI: https://doi.org/10.1016/j.jep.2017.09.009
Shetty S, Udupa S, Udupa L. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of Ocimum sanctum Linn in rats. Evidence-Based Complement Altern Med 2008;5:95-101. DOI: https://doi.org/10.1093/ecam/nem004
Mahoney N, Molyneux RJ, Campbell BC. Regulation of aflatoxin production by naphthoquinones of walnut (Juglans regia). J Agric Food Chem 2000;48:4418-21. DOI: https://doi.org/10.1021/jf0003449
Fernández-Agulló A, Pereira E, Freire MS, et al. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind Crops Prod 2013;42:126-32.
Omidi Ghaleh Mohammadi M, Mirghazanfari SM. Investigation of Iranian pomegranate cultivars for wound healing components. Eur J Transl Myol 2019;29:22-6. DOI: https://doi.org/10.4081/ejtm.2019.7995
Hakkim FL, Shankar CG, Girija S. Chemical Composition and Antioxidant Property of Holy Basil (Ocimum sanctum L.) Leaves, Stems, and Inflorescence and Their in Vitro Callus Cultures. J Agric Food Chem 2007;55:9109-17. DOI: https://doi.org/10.1021/jf071509h
Rice-Evans C. Flavonoid Antioxidants. Curr Med Chem 2001;8:797-807. DOI: https://doi.org/10.2174/0929867013373011
Fernández-Agulló A, Pereira E, Freire MS, et al. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind Crops Prod 2013;42:126-32. DOI: https://doi.org/10.1016/j.indcrop.2012.05.021
Meir S, Kanner J, Akiri B, Philosoph-Hadas S. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem 1995;43:1813-9. DOI: https://doi.org/10.1021/jf00055a012
Oliveira I, Sousa A, Ferreira ICFR, et al. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol 2008;46:2326-31. DOI: https://doi.org/10.1016/j.fct.2008.03.017
Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39:44-84. DOI: https://doi.org/10.1016/j.biocel.2006.07.001
Khorshid F, Ali SS, Alsofyani T, Albar H. Plectranthus tenuiflorus (Shara) Promotes Wound Healing: In vitro and in vivo Studies. Int J Bot 2010;6:69-80. DOI: https://doi.org/10.3923/ijb.2010.69.80
Sheeba M, Emmanuel S, Revathi K, Ignacimuthu S. Wound healing activity of Cassia occidentalis L. in albino Wistar rats. Int J Integr Biol 2009;8:1-6.
Hagh-Nazari L, Goodarzi N, Zangeneh MM, et al. Stereological study of kidney in streptozotocin-induced diabetic mice treated with ethanolic extract of Stevia rebaudiana (bitter fraction). Comp Clin Path 2017;26:455-63. DOI: https://doi.org/10.1007/s00580-016-2398-7
Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care 2014;3:445-64. DOI: https://doi.org/10.1089/wound.2013.0473
Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13:159. DOI: https://doi.org/10.1038/nri3399
Boniakowski AE, Kimball AS, Jacobs BN, et al. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J Immunol 2017;199:17-24. DOI: https://doi.org/10.4049/jimmunol.1700223
desJardins-Park HE, Foster DS, Longaker MT. Fibroblasts and wound healing: an update. Regen Med 2018;13:491-5. DOI: https://doi.org/10.2217/rme-2018-0073
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/ejtm.2019.8671
https://doi.org/10.4081/ejtm.2019.8671



