Reinnervation of Vastus lateralis is increased significantly in seniors (70-years old) with a lifelong history of high-level exercise (2013, revisited here in 2022)
HTML: 22
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
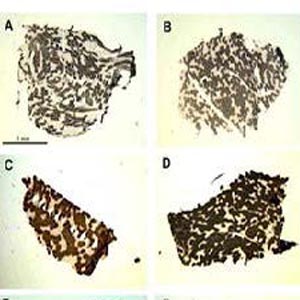
In 2013 we presented results showing that at the histological level lifelong increased physical activity promotes reinnervation of muscle fibers in aging muscles. Indeed, in muscle biopsies from 70-year old men with a lifelong history of high-level physical activity, we observed a considerable increase in fiber-type groupings (F-TG), almost exclusively of the slow type. Slow-type transformation by denervation-reinnervation in senior sportsmen seems to fluctuate from those with scarce fiber-type transformation and groupings to almost fully transformed muscle, going through a process in which isolated fibers co-expressing fast and slow Myosin Heavy Chains (MHCs) seems to fill the gaps. Taken together, our results suggest that, beyond the direct effects of aging on the muscle fibers, changes occurring in skeletal muscle tissue appear to be largely, although not solely, a result of sparse denervation-reinnervation. The lifelong exercise allows the body to adapt to the consequences of the age-related denervation and to preserve muscle structure and function by saving otherwise lost muscle fibers through recruitment to different, mainly slow, motor units. These beneficial effects of high-level life-long exercise on motoneurons, specifically on the slow type motoneurones that are those with higher daily activity, and on muscle fibers, serve to maintain size, structure and function of muscles, delaying the functional decline and loss of independence that are commonly seen in late aging. Several studies of independent reserchers with independent analyses confirmed and cited our 2013 results. Thus, the results we presented in our paper in 2013 seem to have held up rather well.
CURRICULUM VITAE: Prof. UGO CARRARO
ORCID iD: http://orcid.org/0000-0002-0924-4998
Prof. Ugo Carraro, M.D.,
Senior Scholar of the University of Padova, Italy
Department of Biomedical Sciences, University of Padova, Italy
phone: +39 338 1575745
E-mail: ugo.carraro@unipd.it
Born: February 23, 1943, Abano Terme (Padova), Italy
Citizenship: Italy
Degree and Academic Positions
- M.D. (Laurea in Medicina e Chirurgia), University of Padua, Italy -1968
- Associate Professor of General Pathology, Faculty of Medicine, University of Padova,1983-2013
- Principal Investigator of the Laboratory of Applied Myology of the C.N.R Institute of Neuroscience, 1983-2000
- Acting-director of the Department of Biomedical Science -1998 to 2003;
- Principal Investigator of the Translational Myology Lab, Department of Biomedical Science -1998 to 2013
- Interdepartmental Research Center of Myology (cirMYO), Founder and Head 2005 - 2011
Other Professional Activities
- 1991-2019 – Founder and Editor-in-Chief of Basic Applied Myology: 1991 to date
- Consultant of I.R.C.C.S. Ospedale San Camillo di Venezia-Lido, Italy
- Referee for International Journals and Granting Agencies: J Cell Biol, Muscle&Nerve, Artificial Organs, J Muscle Res Cell Motility, Artificial Organs, Cell Death & Differentiation, Annals Thoracic Surgery, Acta Physiologica, The Open Rehabilitation Journal, Association Française contre les Myopathies
- Organizer of International Conferences and Courses
- Invited speaker and chairman in International Conferences
Main Research Interests
- Translational Myology: Basics of muscle plasticity and their applications to medical research, in particular:
- Functional Electrical Stimulation (FES) of denervated human muscle
- Role of regenerative myogenesis in exercise-induced muscle damage and denervation
- Reconstruction, neurotization and artificial synaptogenesis of ablated skeletal muscle
- Analyses in muscle atrophy and apoptosis of role of Cytokines and Myokines by invasive and non-invasive samplings
Achievments and expertises
Prof. Ugo Carraro is a world-class leader in molecular and structural analyses of skeletal muscle. He developed bi-dimensional gel electrophoresis for myosin light chains, in particular the embryonic isoform, and was the first to separate human myosin heavy chain isoforms.
He discovered the long-term potential of denervated muscle to survive denervation by non-compensatory myofiber regeneration.
Prof. Carraro was Associate Professor of General Pathology at the Faculty of Medicine of the University of Padova, from 1983 to 2013. Editor-in-Chief of The European Journal of Translational Myology since 1991, he founded and chaired from 2005 to 2011 Interdepartmental Research Center of Myology (CIR-Myo) of the University of Padova. CIR-Myo continue to join scientists and clinicians of the Departments of Biology, Biomedical Sciences, Neuroscience, Medicine and Surgical Sciences, Physical Medicine and Rehabilitation and Experimental & Clinical Veterinary Sciences.
In collaboration with international partners, in particular the Ludwig Boltzmann Institute of Electrostimulation and Physical Rehabilitation of the Wilheminenspital, Vienna, Austria, CIR-Myo scientists and clinicians developed and implemented the expertise and facilities to maintain and extend in Interreg IVa a world-unique BIO-BANK of human skeletal muscle biopsies harvested from upper motor neuron and lower motor neuron denervated patients and related animal research, young and senior sportsmen, healthy and diseased elderly persons before and during recovery by new therapies and rehabilitation strategies. In particular, muscle biopsies were harvested from patients affected with spinal cord injury and severe leg trauma, osteoarthropathies and rheumatic autoimmune diseases, and cancer.
CIR-Myo is developing also new imaging methods for functional monitoring of human skeletal muscles from patients suffering with permanent and transient muscle denervation. The collaboration with the Dr. Kern’s Vienna Group resulted in new knowledge and clinical validation of rehabilitation strategies for permanently denervated human muscles using home-based Functional Electrical Stimulation (FES). Thus, a world unique human muscle biopsies DATA-BASE of structural and molecular data obtained by histology, histo- and immuno-chemistry, electron microscopy and genomic/proteomic approaches is available to compare new rehabilitation strategies against standard clinical methods.
Carraro’s Lab matured expertises working on different aspects of muscle biology and pathology, including spinal cord injuries, aging, apoptosis, and muscle regeneration. This full set of methods and expertise are uniquely present at the CIR-Myo of Padova University, and well documented by a list of original results published in leading Journals of the different research fields.
Now Professor Carraro is validating NON-INVASIVE BLOOD ANALYSES to monitor Cytokines (anti- and pro- Inflammatory) and Myokines by saliva and sweat samplings, a very promising approach that will increase acceptability of sampling by volunteering persons and frequency of sampling, a key factor to evaluate the many very transient effects of trainings and rehabilitations in early aging and aging.
Publications
PUBMED April 12, 2020: Ugo Carraro 1973 - 2019 - References: 156 - Citations > 3000 –
h-index 44; h-index 37, excluding self-citations
List of 10 recent publications 2020-2016
- Ricciardi C, Edmunds KJ, Recenti M, Sigurdsson S, Gudnason V, Carraro U, Gargiulo P. Assessing cardiovascular risks from a mid-thigh CT image: a tree-based machine learning approach using radiodensitometric distributions. Sci Rep. 2020 Feb 18;10(1):2863. doi: 10.1038/s41598-020-59873-9. PMID: 32071412
- Carraro U. 2020PMD, 30-years of Translational Mobility Medicine at the time of COVID-19 outbreak: Last-minute forewords from the editor. Eur J Transl Myol 2020;30:8966. Doi 10.4081/ejtm.2019.8966.
- Albertin G, Ravara B, Kern H, Hofer C, Loefler S, Jurecka W, Guidolin D, Rambaldo A, Porzionato A, De Caro R, Zampieri S, Pond A, Alaibac M, Carraro U. Two-years of home based functional electrical stimulation recovers epidermis from atrophy and flattening after years of complete Conus-Cauda Syndrome. Medicine (Baltimore). 2019 Dec;98(52):e18509. doi: 10.1097/MD.0000000000018509. PMID: 31876739.
- Kern H, Gargiulo P, Pond A, Albertin G, Marcante A, Carraro U. To Reverse Atrophy of Human Muscles in Complete SCI Lower Motor Neuron Denervation by Home-Based Functional Electrical Stimulation. Adv Exp Med Biol. 2018;1088:585-591. doi: 10.1007/978-981-13-1435-3_27.
- Carraro U, Gava K, Baba A, Marcante A, Piccione F. To Contrast and Reverse Skeletal Muscle Atrophy by Full-Body In-Bed Gym, a Mandatory Lifestyle for Older Olds and Borderline Mobility-Impaired Persons. Adv Exp Med Biol. 2018;1088:549-560. doi: 10.1007/978-981-13-1435-3_25. Review.
- Carraro U. Exciting perspectives for Translational Myology in the Abstracts of the 2018Spring PaduaMuscleDays: Giovanni Salviati Memorial - Chapter I - Foreword. Eur J Transl Myol. 2018 Feb 20;28(1):7363. doi: 10.4081/ejtm.2018.7363. eCollection 2018 Jan 12.
- Mosole S, Zampieri S, Furlan S, Carraro U, Löefler S, Kern H, Volpe P, Nori A. Effects of Electrical Stimulation on Skeletal Muscle of Old Sedentary People. Gerontol Geriatr Med. 2018 Apr 10;4:2333721418768998. doi: 10.1177/2333721418768998. eCollection 2018 Jan-Dec.
- Edmunds K, Gíslason M, Sigurðsson S, Guðnason V, Harris T, Carraro U, Gargiulo P. Advanced quantitative methods in correlating sarcopenic muscle degeneration with lower extremity function biometrics and comorbidities. PLoS One. 2018 Mar 7;13(3):e0193241. doi: 10.1371/journal.pone.0193241. eCollection 2018.
- Atrophy, ultra-structural disorders, severe atrophy and degeneration of denervated human muscle in SCI and Kern H, Hofer C, Loefler S, Zampieri S, Gargiulo P, Baba A, Marcante A, Piccione F, Pond A, Carraro U. Aging. Implications for their recovery by Functional Electrical Stimulation, updated 2017. Neurol Res. 2017 Jul;39(7):660-666. doi: 10.1080/01616412.2017.1314906. Epub 2017 Apr 13. Review.
- Edmunds KJ, Árnadóttir Í, Gíslason MK, Carraro U, Gargiulo P. Nonlinear Trimodal Regression Analysis of Radiodensitometric Distributions to Quantify Sarcopenic and Sequelae Muscle Degeneration. Comput Math Methods Med. 2016;2016:8932950. doi: 10.1155/2016/8932950. Epub 2016 Dec 27.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.
Similar Articles
- Simone Mosole, Katia Rossini, Helmut Kern, Stefan Löfler, Hannah Fruhmann, Michael Vogelauer, Samantha Burggraf, Martina Grim-Stieger, Ján Cvečka, Dušan Hamar, Milan Sedliak, Nejc Šarabon, Amber Pond, Donatella Biral, Ugo Carraro, Sandra Zampieri, Reinnervation of Vastus lateralis is increased significantly in seniors (70-years old) with a lifelong history of high-level exercise , European Journal of Translational Myology: Vol. 23 No. 4 (2013)
- Simone Mosole, Ugo Carraro, Helmut Kern, Stefan Loefler, Sandra Zampieri, Use it or lose it: tonic activity of slow motoneurons promotes their survival and preferentially increases slow fiber-type groupings in muscles of old lifelong recreational sportsmen , European Journal of Translational Myology: Vol. 26 No. 4 (2016)
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/ejtm.2022.10420
https://doi.org/10.4081/ejtm.2022.10420



