Magnetic resonance imaging based muscle strain rate mapping during eccentric contraction to study effects of unloading induced by unilateral limb suspension
HTML: 11
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
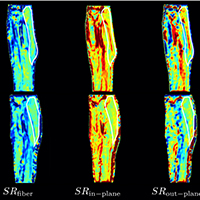
Age- and disuse- related loss of muscle force is disproportionately larger than the loss of muscle mass. Earlier studies reported that comparing concentric and eccentric contractions, there is a significant age-related decrease in force only in concentric contractions. Magnetic Resonance Imaging enables mapping of muscle deformation and has been used to study isometric but not eccentric contractions. We report MRI based strain rate mapping of the medial gastrocnemius in subjects pre- and post-unloading induced by Unilateral Limb Suspension. In contrast to isometric contraction, no difference in strain rate indices were observed post-unloading, in conformance with preserved force during eccentric contractions.
Bodine SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol. 2013;45:2200–8. DOI: https://doi.org/10.1016/j.biocel.2013.06.011
Bongers KS, Fox DK, Kunkel SD, et. al. Spermine oxidase maintains basal skeletal muscle gene expression and fiber size and is strongly repressed by conditions that cause skeletal muscle atrophy. American Journal of Physiology-Endocrinology and Metabolism. 2015;308:E144–58. DOI: https://doi.org/10.1152/ajpendo.00472.2014
Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. Journal of Applied Physiology. 1990;68:1–12. DOI: https://doi.org/10.1152/jappl.1990.68.1.1
de Boer MD, Maganaris CN, Seynnes OR, et. al. Time course of muscular, neural and tendinous adaptations to 23 day unilateral lower-limb suspension in young men. The Journal of Physiology. 2007;583:1079–91. DOI: https://doi.org/10.1113/jphysiol.2007.135392
de Boer MD, Selby A, Smith K, et. al. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. The Journal of Physiology 2007;585:241–51. DOI: https://doi.org/10.1113/jphysiol.2007.142828
Lieber RL, Ward SR. Cellular mechanisms of tissue fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am J Physiol, Cell Physiol. 2013;305:C241–52. DOI: https://doi.org/10.1152/ajpcell.00173.2013
Ramaswamy KS, Palmer ML, van der Meulen JH, et. al. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. The Journal of Physiology. 2011;589:1195–208. DOI: https://doi.org/10.1113/jphysiol.2010.201921
Sharafi B, Blemker SS. A mathematical model of force transmission from intrafascicularly terminating muscle fibers. Journal of Biomechanics. 2011;44:2031–9. DOI: https://doi.org/10.1016/j.jbiomech.2011.04.038
Bilston LE, Bolsterlee B, Nordez A, Sinha S. Contemporary image-based methods for measuring passive mechanical properties of skeletal muscles in vivo. J Appl Physiol. 2019;126:1454–64. DOI: https://doi.org/10.1152/japplphysiol.00672.2018
Barber LA, Barrett RS, Gillett JG, et al. Neuromechanical properties of the triceps surae in young and older adults. Exp Gerontol. 2013;48:1147–55. DOI: https://doi.org/10.1016/j.exger.2013.07.007
Roig M, MacIntyre DL, Eng JJ, Reid WD. Preservation of eccentric strength in older adults: Evidence, mechanisms and implications for training and rehabilitation. Exp Gerontol. 2010 Jun;45(6):400–9. DOI: https://doi.org/10.1016/j.exger.2010.03.008
Malis V, Sinha U, Csapo R, et al. Relationship of changes in strain rate indices estimated from velocity-encoded MR imaging to loss of muscle force following disuse atrophy. Magn Reson Med. 2018;79:912–22. DOI: https://doi.org/10.1002/mrm.26759
Zhang Y, Chen J-S, He Q, He X, et al. Microstructural analysis of skeletal muscle force generation during aging. Int J Numer Meth Biomed Engng. 2019;52:B125. DOI: https://doi.org/10.1002/cnm.3295
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.


 https://doi.org/10.4081/ejtm.2019.8935
https://doi.org/10.4081/ejtm.2019.8935



